化工学报 ›› 2021, Vol. 72 ›› Issue (6): 3031-3040.DOI: 10.11949/0438-1157.20201705
收稿日期:2020-11-30
修回日期:2021-03-01
出版日期:2021-06-05
发布日期:2021-06-05
通讯作者:
方莉
作者简介:张少博(1996—),男,硕士研究生,基金资助:
ZHANG Shaobo1,2( ),FANG Li1,2(
),FANG Li1,2( ),GAO Xuetao1,2,CHENG Wenting2
),GAO Xuetao1,2,CHENG Wenting2
Received:2020-11-30
Revised:2021-03-01
Online:2021-06-05
Published:2021-06-05
Contact:
FANG Li
摘要:
以氯化镁、氢氧化钠和硫酸镁为原料,采用溶胶-凝胶-水热法制备了碱式硫酸镁(MHSH-512)晶须,研究了前驱物Mg(OH)2、Mg2+和
中图分类号:
张少博, 方莉, 高雪焘, 程文婷. 碱式硫酸镁晶须的可控制备及不同离子的影响机制[J]. 化工学报, 2021, 72(6): 3031-3040.
ZHANG Shaobo, FANG Li, GAO Xuetao, CHENG Wenting. Controllable synthesis of magnesium hydroxide sulfate hydrate whiskers and effects of different ions[J]. CIESC Journal, 2021, 72(6): 3031-3040.

图7 不同水热时间去除Cl-前、后合成的MHSH-512晶须的SEM图[c(Mg2+)/c(SO42-)=3, 190℃]
Fig.7 SEM images of MHSH-512 whiskers synthesized before and after removal of Cl– with different hydrothermal treatment time
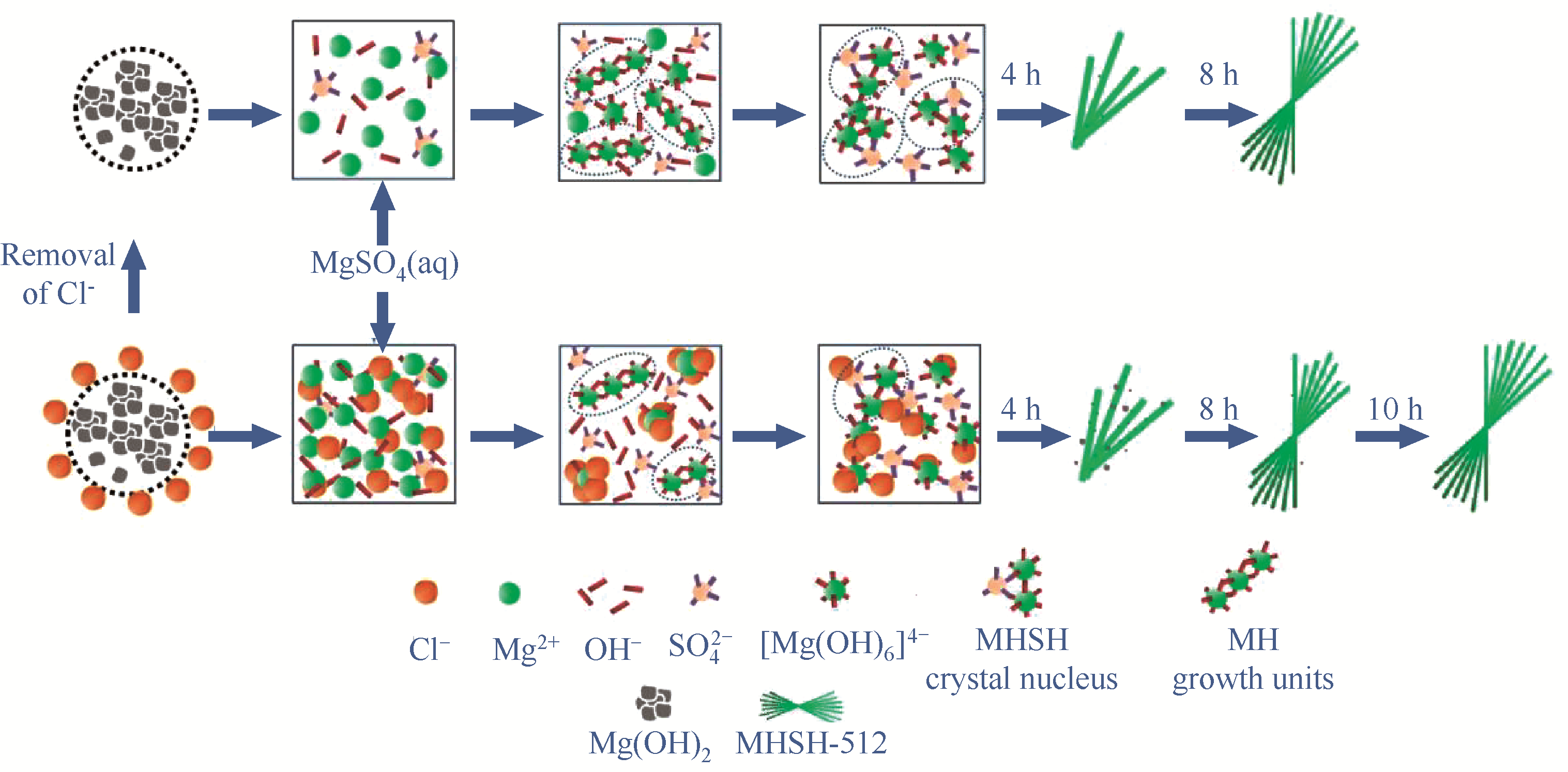
图8 去除Cl–前、后MHSH-512晶须的形成与生长机理示意图
Fig.8 A schematic diagram of the formation and growth mechanisms of MHSH-512 whiskers before and after removal of Cl–
| 1 | Wu L, Jin Z, Zhang Z. Application and synthesis of inorganic whisker materials [J]. Progress in Chemistry, 2003, 15(4): 264-274. |
| 2 | Yang L, Shi C L. Effect of zinc hydroxystannate coated M-HOS whisker on flame retardant properties of flexible PVC[J]. Procedia Engineering, 2018, 211: 901-905. |
| 3 | Xu H H, Smith D T, Schumacher G E, et al. Whisker-reinforced dental core buildup composites: effect of filler level on mechanical properties[J]. Journal of Biomedical Materials Research, 2000, 52(4): 812-818. |
| 4 | 吴健松, 梁海群. 人工可控氢氧化镁晶须生长[J]. 人工晶体学报, 2013, 42(2): 299-304. |
| Wu J S, Liang H Q. Artificial controlled crystal growth of magnesium hydroxide whisker[J]. Journal of Synthetic Crystals, 2013, 42(2): 299-304. | |
| 5 | Yue T, Gao S Y, Zhu L X, et al. Crystal growth and crystal structure of magnesium oxysulfate 2MgSO4·Mg(OH)2·2H2O[J]. Journal of Molecular Structure, 2002, 616(1/2/3): 247-252. |
| 6 | Li J G, Wang F F, Zhang Y J, et al. Microstructure and mechanical properties of magnesium matrix composite reinforced with magnesium borate whisker[J]. Journal of Composite Materials, 2012, 46(24): 3011-3016. |
| 7 | Shen R, Chu G, Shen X P. Advances research on preparation of magnesium carbonate whisker[J]. Advanced Materials Research, 2013, 699: 17-21. |
| 8 | Sain M, Park S H, Suhara F, et al. Flame retardant and mechanical properties of natural fibre-PP composites containing magnesium hydroxide[J]. Polymer Degradation and Stability, 2004, 83(2): 363-367. |
| 9 | Chiu S H, Wang W K. The dynamic flammability and toxicity of magnesium hydroxide filled intumescent fire retardant polypropylene[J]. Journal of Applied Polymer Science, 1998, 67(6): 989-995. |
| 10 | Lu H D, Hu Y, Yang L, et al. Study of the fire performance of magnesium hydroxide sulfate hydrate whisker flame retardant polyethylene[J]. Macromolecular Materials and Engineering, 2004, 289(11): 984-989. |
| 11 | Gao C H, Li X G, Feng L J, et al. Preparation and thermal decomposition of 5Mg(OH)2·MgSO4·2H2O nanowhiskers[J]. Chemical Engineering Journal, 2009, 150(2/3): 551-554. |
| 12 | Zhang J J, Wu B, Tao S, et al. Hydrothermal synthesis of magnesium hydroxide sulfate hydrate whisker flame retardant[J]. Applied Mechanics and Materials, 2012, 174/175/176/177: 1034-1037. |
| 13 | 吴健松, 肖应凯, 梁海群, 等. 丁醇-变频微波-水热法制备优质碱式硫酸镁晶须及表征[J]. 人工晶体学报, 2009, 38(1): 285-289. |
| Wu J S, Xiao Y K, Liang H Q, et al. Butanol-frequency microwave-hydrothermal synthesis and characterization of high-quality hydrous magnesium oxysulfate whiskers[J]. Journal of Synthetic Crystals, 2009, 38(1): 285-289. | |
| 14 | Ma X L, Ning G Q, Qi C L, et al. One-step synthesis of basic magnesium sulfate whiskers by atmospheric pressure reflux[J]. Particuology, 2016, 24: 191-196. |
| 15 | Tang Z L, Zhu C L, Fan F Y, et al. Green synthesis of the excellent magnesium oxysulfate whiskers under controlled reaction conditions[J]. Materials Chemistry and Physics, 2017, 195: 143-148. |
| 16 | 高传慧, 许军, 王传兴, 等. 碱式硫酸镁晶须的一步法水热合成及生长机理[J]. 硅酸盐学报, 2011, 39(5): 773-778. |
| Gao C H, Xu J, Wang C X, et al. Hydrothermal synthesis and growth mechanism of magnesium hydroxide sulfate hydrate whiskers by the one-step procedure[J]. Journal of the Chinese Ceramic Society, 2011, 39(5): 773-778. | |
| 17 | 岳涛, 高世扬, 朱黎霞, 等. 5Mg(OH)2·MgSO4·2H2O晶须生长过程中的形貌研究[J]. 无机化学学报, 2002, 18(3): 313-316. |
| Yue T, Gao S Y, Zhu L X, et al. Study on the morphology of 5Mg(OH)2·MgSO4·2H2O in whisker growth process[J]. Chinese Journal of Inorganic Chemistry, 2002, 18(3): 313-316. | |
| 18 | Xiang L, Liu F, Li J, et al. Hydrothermal formation and characterization of magnesium oxysulfate whiskers[J]. Materials Chemistry and Physics, 2004, 87(2/3): 424-429. |
| 19 | Li J, Xiang L, Jin Y. Hydrothermal formation of magnesium oxysulfate whiskers in the presence of ethylenediaminetetraacetic acid[J]. Journal of Materials Science, 2006, 41(5): 1345-1348. |
| 20 | Yan X X, Xu D L, Xue D F. SO42- ions direct the one-dimensional growth of 5Mg(OH)2·MgSO4·2H2O[J]. Acta Materialia, 2007, 55(17): 5747-5757. |
| 21 | Gao C H, Ding L, Wu Y M, et al. Low-cost synthesis and morphology control of magnesium oxysulfate hydrate whiskers[J]. Advanced Materials Research, 2014, 936: 986-991. |
| 22 | 乌志明. 前驱物分解法介观形貌镁质材料研究[D]. 西宁: 中国科学院研究生院(青海盐湖研究所), 2006. |
| Wu Z M. Studies on mesoscopic morphological magnesia materials with precursor decomposition method[D]. Xining: Graduate School of Chinese Academy of Sciences (Qinghai Salt Lake Institute), 2006. | |
| 23 | Ding Y, Zhang G T, Zhang S Y, et al. Preparation and characterization of magnesium hydroxide sulfate hydrate whiskers[J]. Chemistry of Materials, 2000, 12(10): 2845-2852. |
| 24 | Yu R, Pee J H, Kim H T, et al. Effect of MgO and NH4OH on formation of 5Mg(OH)2·MgSO4·3H2O whiskers[J]. Key Engineering Materials, 2012, 512/513/514/515: 91-94. |
| 25 | 黄虹. 塑料成型加工与模具[M]. 2版. 北京: 化学工业出版社, 2009: 82-101. |
| Huang H. Plastic Molding Process and Mold [M]. 2nd ed. Beijing: Chemical Industry Press, 2009: 82-101. | |
| 26 | Yang Y F, Wu X F, Hu G S, et al. Effects of stearic acid on synthesis of magnesium hydroxide via direct precipitation[J]. Journal of Crystal Growth, 2008, 310(15): 3557-3560. |
| 27 | Dinnebier R E, Pannach M, Freyer D. 3Mg(OH)2·MgSO4·8H2O: a metastable phase in the system Mg(OH)2-MgSO4-H2O[J]. Zeitschrift Für Anorganische und Allgemeine Chemie, 2013, 639(10): 1827-1833. |
| 28 | Sun X T, Xiang L. Synthesis of magnesium oxysulfate whiskers in the presence of sodium dodecyl benzene sulfonate[J]. Crystal Research and Technology, 2008, 43(5): 479-482. |
| 29 | 向兰, 金永成, 金涌. 氢氧化镁的结晶习性研究[J]. 无机化学学报, 2003, 19(8): 837-842. |
| Xiang L, Jin Y C, Jin Y. Study on the growth of magnesium hydroxide crystals[J]. Chinese Journal of Inorganic Chemistry, 2003, 19(8): 837-842. | |
| 30 | Hamada E, Ishizawa N, Marumo F, et al. Structure of Mg6SO2(OH)14 determined by micro single-crystal X-ray diffraction[J]. Acta Crystallographica Section B, 1996, 52(2): 266-269. |
| 31 | Fainberg A H, Winstein S. Salt effects and ion pairs in solvolysis and related reactions(Ⅳ): Salt effects in acetolysis of neophyl and p-methoxyneophyl halides and arylsulfonates[J]. Journal of the American Chemical Society, 1956, 78(12): 2763-2767. |
| 32 | 张颖, 蒋明学, 张军战. 合成温度对碳热还原法合成碳化硅晶须形貌的影响[J]. 人工晶体学报, 2010, 39(2): 369-374. |
| Zhang Y, Jiang M X, Zhang J Z. Effect of synthesis temperature on morphology of SiC whiskers by carbothermal reduction method[J]. Journal of Synthetic Crystals, 2010, 39(2): 369-374. |
| [1] | 王琪, 张斌, 张晓昕, 武虎建, 战海涛, 王涛. 氯铝酸-三乙胺离子液体/P2O5催化合成伊索克酸和2-乙基蒽醌[J]. 化工学报, 2023, 74(S1): 245-249. |
| [2] | 车睿敏, 郑文秋, 王小宇, 李鑫, 许凤. 基于离子液体的纤维素均相加工研究进展[J]. 化工学报, 2023, 74(9): 3615-3627. |
| [3] | 赵亚欣, 张雪芹, 王荣柱, 孙国, 姚善泾, 林东强. 流穿模式离子交换层析去除单抗聚集体[J]. 化工学报, 2023, 74(9): 3879-3887. |
| [4] | 陆俊凤, 孙怀宇, 王艳磊, 何宏艳. 离子液体界面极化及其调控氢键性质的分子机理[J]. 化工学报, 2023, 74(9): 3665-3680. |
| [5] | 郑佳丽, 李志会, 赵新强, 王延吉. 离子液体催化合成2-氰基呋喃反应动力学研究[J]. 化工学报, 2023, 74(9): 3708-3715. |
| [6] | 宋明昊, 赵霏, 刘淑晴, 李国选, 杨声, 雷志刚. 离子液体脱除模拟油中挥发酚的多尺度模拟与研究[J]. 化工学报, 2023, 74(9): 3654-3664. |
| [7] | 王阳, 戴永强, 曾炜. 2,5-二羟基苯磺酸增强离子水凝胶材料热电性能的研究[J]. 化工学报, 2023, 74(9): 3946-3955. |
| [8] | 杨绍旗, 赵淑蘅, 陈伦刚, 王晨光, 胡建军, 周清, 马隆龙. Raney镍-质子型离子液体体系催化木质素平台分子加氢脱氧制备烷烃[J]. 化工学报, 2023, 74(9): 3697-3707. |
| [9] | 陈美思, 陈威达, 李鑫垚, 李尚予, 吴有庭, 张锋, 张志炳. 硅基离子液体微颗粒强化气体捕集与转化的研究进展[J]. 化工学报, 2023, 74(9): 3628-3639. |
| [10] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [11] | 王俐智, 杭钱程, 郑叶玲, 丁延, 陈家继, 叶青, 李进龙. 离子液体萃取剂萃取精馏分离丙酸甲酯+甲醇共沸物[J]. 化工学报, 2023, 74(9): 3731-3741. |
| [12] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [13] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [14] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [15] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号