化工学报 ›› 2020, Vol. 71 ›› Issue (11): 5052-5058.DOI: 10.11949/0438-1157.20200884
杨小青1,2( ),廖泉飞1,2,易芸1,2,杨春亮1,2,赵天翔1,2(
),廖泉飞1,2,易芸1,2,杨春亮1,2,赵天翔1,2( ),胡兴邦3,刘飞1,2
),胡兴邦3,刘飞1,2
收稿日期:2020-07-03
修回日期:2020-08-16
出版日期:2020-11-05
发布日期:2020-11-05
通讯作者:
赵天翔
作者简介:杨小青(1996—),女,硕士研究生,基金资助:
Xiaoqing YANG1,2( ),Quanfei LIAO1,2,Yun YI1,2,Chunliang YANG1,2,Tianxiang ZHAO1,2(
),Quanfei LIAO1,2,Yun YI1,2,Chunliang YANG1,2,Tianxiang ZHAO1,2( ),Xingbang HU3,Fei LIU1,2
),Xingbang HU3,Fei LIU1,2
Received:2020-07-03
Revised:2020-08-16
Online:2020-11-05
Published:2020-11-05
Contact:
Tianxiang ZHAO
摘要:
多羟基醇如丙三醇、乙二醇、聚乙二醇等富含氢键位点,通过氢键相互作用能循环捕集SO2气体。然而,羟基比例更高的丙三醇和乙二醇对SO2的吸收能力远弱于聚乙二醇,这表明除氢键作用之外,可能还存在SO2与醚氧原子间的电荷转移作用。为了证实SO2与醚氧原子间的电荷转移作用,实验选择沸点较高的三乙二醇二甲醚作为SO2的吸收剂。首先采用气体动态单循环法测定了低浓度SO2(体积分数为2010 × 10-6)在122.7 kPa和293.15~313.15 K下的汽液相平衡数据,并且基于汽液平衡数据,计算了SO2吸收过程的热力学参数,包括亨利常数、熵变、焓变和Gibbs自由能等。此外,基于紫外光谱、红外光谱和核磁波谱的实验结果,证实了SO2和三乙二醇二甲醚之间的O…S电荷转移相互作用。
中图分类号:
杨小青,廖泉飞,易芸,杨春亮,赵天翔,胡兴邦,刘飞. 三乙二醇二甲醚吸收低浓度SO2性能及机理研究[J]. 化工学报, 2020, 71(11): 5052-5058.
Xiaoqing YANG,Quanfei LIAO,Yun YI,Chunliang YANG,Tianxiang ZHAO,Xingbang HU,Fei LIU. Study on performance and mechanism of triethylene glycol dimethyl ether for capturing low concentration SO2[J]. CIESC Journal, 2020, 71(11): 5052-5058.
| T/K | |||
|---|---|---|---|
| 293.15 | 6.83 | 16.5 | 134 |
| 12.95 | 30.2 | 246 | |
| 18.05 | 40.8 | 333 | |
| 22.44 | 52.0 | 425 | |
| 31.31 | 70.7 | 577 | |
| 35.55 | 79.5 | 649 | |
| 45.19 | 101.9 | 832 | |
| 298.15 | 8.87 | 25.5 | 208 |
| 12.90 | 36.6 | 298 | |
| 17.85 | 49.7 | 406 | |
| 22.95 | 63.1 | 515 | |
| 29.12 | 80.6 | 658 | |
| 33.00 | 92.5 | 755 | |
| 37.59 | 106.3 | 867 | |
| 303.15 | 1.91 | 13.6 | 111 |
| 3.11 | 18.5 | 151 | |
| 6.42 | 31.2 | 254 | |
| 10.56 | 48.3 | 395 | |
| 14.90 | 63.8 | 521 | |
| 17.70 | 75.7 | 618 | |
| 25.77 | 105.0 | 857 | |
| 308.15 | 2.74 | 13.1 | 107 |
| 5.07 | 25.8 | 210 | |
| 7.14 | 36.2 | 295 | |
| 10.25 | 50.7 | 414 | |
| 14.23 | 70.5 | 576 | |
| 16.30 | 80.0 | 653 | |
| 21.37 | 106.1 | 866 | |
| 313.15 | 1.45 | 10.9 | 89 |
| 4.50 | 33.1 | 270 | |
| 7.14 | 49.3 | 403 | |
| 8.38 | 58.1 | 474 | |
| 10.82 | 71.2 | 581 | |
| 13.87 | 92.5 | 755 | |
| 15.06 | 100.2 | 818 |
表1 不同温度下三乙二醇二甲醚吸收低浓度SO2的汽液相平衡数据
Table 1 Gas-liquid equilibrium data for dilute SO2 in triethylene glycol dimethyl ether at different temperature
| T/K | |||
|---|---|---|---|
| 293.15 | 6.83 | 16.5 | 134 |
| 12.95 | 30.2 | 246 | |
| 18.05 | 40.8 | 333 | |
| 22.44 | 52.0 | 425 | |
| 31.31 | 70.7 | 577 | |
| 35.55 | 79.5 | 649 | |
| 45.19 | 101.9 | 832 | |
| 298.15 | 8.87 | 25.5 | 208 |
| 12.90 | 36.6 | 298 | |
| 17.85 | 49.7 | 406 | |
| 22.95 | 63.1 | 515 | |
| 29.12 | 80.6 | 658 | |
| 33.00 | 92.5 | 755 | |
| 37.59 | 106.3 | 867 | |
| 303.15 | 1.91 | 13.6 | 111 |
| 3.11 | 18.5 | 151 | |
| 6.42 | 31.2 | 254 | |
| 10.56 | 48.3 | 395 | |
| 14.90 | 63.8 | 521 | |
| 17.70 | 75.7 | 618 | |
| 25.77 | 105.0 | 857 | |
| 308.15 | 2.74 | 13.1 | 107 |
| 5.07 | 25.8 | 210 | |
| 7.14 | 36.2 | 295 | |
| 10.25 | 50.7 | 414 | |
| 14.23 | 70.5 | 576 | |
| 16.30 | 80.0 | 653 | |
| 21.37 | 106.1 | 866 | |
| 313.15 | 1.45 | 10.9 | 89 |
| 4.50 | 33.1 | 270 | |
| 7.14 | 49.3 | 403 | |
| 8.38 | 58.1 | 474 | |
| 10.82 | 71.2 | 581 | |
| 13.87 | 92.5 | 755 | |
| 15.06 | 100.2 | 818 |
| T/K | Hx/ (Pa·m3·mol-1) | ΔH/ (kJ·mol-1) | ΔS/ (J·mol-1·K-1) | ΔG/ (kJ·mol-1) |
|---|---|---|---|---|
| 293.15 | 2.22 ± 0.05 | -37.16 | -74.08 | -15.45 |
| 298.15 | 2.80 ± 0.03 | -15.08 | ||
| 303.15 | 3.84 ± 0.04 | -14.71 | ||
| 308.15 | 4.96 ± 0.07 | -14.34 | ||
| 313.15 | 6.47 ± 0.10 | -13.97 |
表2 三乙二醇二甲醚吸收低浓度SO2气体的亨利常数、吸收焓变、吸收熵变和Gibbs自由能变
Table 2 Henry’s law constants, absorption enthalpy changes and entropy, and Gibbs free energy changes of dilute SO2 in triethylene glycol dimethyl ether
| T/K | Hx/ (Pa·m3·mol-1) | ΔH/ (kJ·mol-1) | ΔS/ (J·mol-1·K-1) | ΔG/ (kJ·mol-1) |
|---|---|---|---|---|
| 293.15 | 2.22 ± 0.05 | -37.16 | -74.08 | -15.45 |
| 298.15 | 2.80 ± 0.03 | -15.08 | ||
| 303.15 | 3.84 ± 0.04 | -14.71 | ||
| 308.15 | 4.96 ± 0.07 | -14.34 | ||
| 313.15 | 6.47 ± 0.10 | -13.97 |
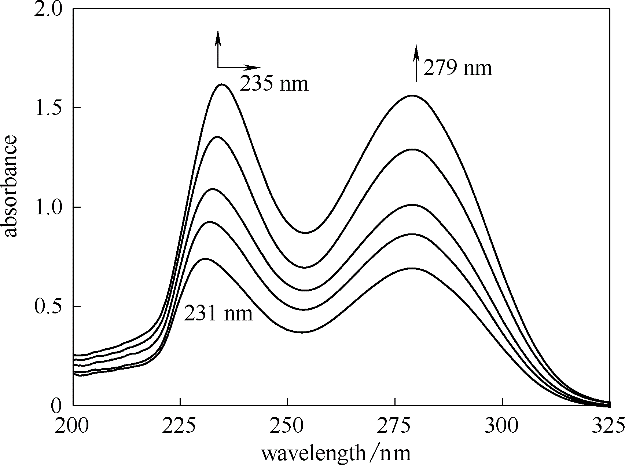
图7 三乙二醇二甲醚 + SO2的紫外光谱图(变化趋势随SO2浓度增大)
Fig.7 UV-Vis spectra of triethylene glycol dimethyl ether + SO2 with the change trend of increased concentration of SO2
| 1 | Smith S J, Aardenn J, Klimont Z, et al. Anthropogenic sulfur dioxide emissions[J]. Atmos. Chem. Phys., 2011, 11: 1101-1116. |
| 2 | Koralegedara N H, Pinto P X, Dionysiou D D, et al. Recent advances in flue gas desulfurization gypsum processes and applications—a review[J]. J. Environ. Manage., 2019, 251(1): 109572. |
| 3 | Ma X X, Kaneko T, Tashimo T, et al. Use of limestone for SO2 removal from flue gas in the semidry FGD process with a powder-particle spouted bed[J]. Chem. Eng. Sci., 2000, 55(20): 4643-4652. |
| 4 | Yan S R, Han F, Hou Q N, et al. Recent advances in ionic liquid-mediated SO2 capture[J]. Ind. Eng. Chem. Res., 2019, 58(31): 13804-13818. |
| 5 | Ren S H, Hou Y C, Zhang K, et al. Ionic liquids: functionalization and absorption of SO2[J]. Green Energ. Environ., 2018, 3(3): 179-190. |
| 6 | Lei Z G, Dai C N, Chen B H. Gas solubility in ionic liquids[J]. Chem. Rev., 2014, 114(2): 1289-1326. |
| 7 | Long G C, Yang C L, Yang X Q, et al. Bisazole-based deep eutectic solvents for the efficient SO2 absorption and conversion without any additives[J]. ACS Sustainable Chem. Eng., 2020, 8(7): 2608-2613. |
| 8 | Zhao T X, Liang J, Zhang Y T, et al. Unexpectedly efficient SO2 capture and conversion to sulfur in novel imidazole-based deep eutectic solvents[J]. Chem. Commun., 2018, 54(65): 8964-8967. |
| 9 | 邓晓霞, 龚磊, 刘小棒, 等. 咪唑类三元低共熔溶剂捕集低压SO2的实验研究[J]. 化工学报, 2020, 71(1): 368-375. |
| Deng X X, Gong L, Liu X B, et al. Study on the capture of low pressure SO2 by imidazole-based ternary deep eutectic solvents[J]. CIESC Journal, 2020, 71(1): 368-375. | |
| 10 | Hong S Y, Im J, Palgunadi J, et al. Ether-functionalized ionic liquids as highly efficient SO2 absorbents[J]. Energy Environ. Sci., 2011, 4(5): 1802-1806. |
| 11 | 崔国凯, 赵宁, 张峰涛, 等. 离子液体捕集二氧化硫气体的研究进展[J]. 科学通报, 2016, 61(28/29): 3115-3126. |
| Gui G K, Zhao N, Zhang F T, et al. Progress in SO2 capture by ionic liquids [J]. Chinese Sci. Bull., 2016, 61(28/29): 3115-3126. | |
| 12 | Sun S Y, Niu Y X, Sun Z C, et al. Solubility properties and spectral characterization of sulfur dioxide in ethylene glycol derivatives[J]. RSC Adv., 2015, 5(12): 8706-8712. |
| 13 | Jiang Y, Liu X, Deng D. Solubility and thermodynamic properties of SO2 in three low volatile urea derivatives[J]. J. Chem. Thermodyn., 2016, 101: 12-18. |
| 14 | Li H, Liu D Z, Wang F A. Solubility of dilute SO2 in dimethyl sulfoxide [J]. J. Chem. Eng. Data, 2002, 47(4): 772-775. |
| 15 | Huang K, Xia S, Zhang M X, et al. Comparative study of the solubilities of SO2 in five low volatile organic solvents (sulfolane, ethylene glycol, propylene carbonate, N-methylimidazole, and and N-methylpyrrolidone)[J]. J. Chem. Eng. Data, 2014, 59(4): 1202-1212. |
| 16 | Zhang J B, Zhang P Y, Han F, et al. Hydrogen bonding and interaction in the absorption processes of sulfur dioxide in ethylene glycol + water binary desulfurization system[J]. Ind. Eng. Chem. Res., 2009, 48(3): 1287-1291. |
| 17 | Zhang J B, Han F, Wei X H, et al. Spectral studies of hydrogen bonding and interaction in the absorption processes of sulfur dioxide in poly(ethylene glycol) 400 + water binary system[J]. Ind. Eng. Chem. Res., 2010, 49(5): 2025-2030. |
| 18 | He Z Q, Liu J R, Lan D W, et al. Absorption properties and spectroscopic studies of dilute sulfur dioxide in aqueous glycerol solutions[J]. Ind. Eng. Chem. Res., 2012, 51(43): 13882-13890. |
| 19 | Niu Y X, Gao F, Sun S Y, et al. Solubility of dilute SO2 in 1,4-dioxane, 15-crown-5 ether, polyethylene glycol 200, polyethylene glycol 300, and their binary mixtures at 308.15 K and 122.66 kPa[J]. Fluid Phase Equilibr., 2013, 344: 65-70. |
| 20 | Sun S Y, Xu Q, Lan G J, et al. Solubility of dilute sulfur dioxide in binary mixtures of ethylene glycol and tetraethylene glycol dimethyl ether[J]. Fluid Phase Equilibr., 2015, 394: 12-18. |
| 21 | Zhao T X, Sha F, Xiao J B, et al. Absorption, desorption, and spectroscopic investigation of sulfur dioxide in ethylene glycol + dimethyl sulfoxide system[J]. Fluid Phase Equilibr., 2015, 405: 7-16. |
| 22 | Zhao T X, Feng X, Zheng W T, et al. Solubility of dilute SO2 in binary system of polyethylene glycol 200 and dimethyl sulfoxide as a function of liquid composition and systems spectroscopic studies[J]. J. Mol. Liq., 2017, 225: 151-159. |
| 23 | Zhao T X, Li Y F, Zhang Y T, et al. Efficient SO2 capture and fixation to cyclic sulfites by dual ether-functionalized protic ionic liquids without any additives[J]. ACS Sustainable Chem. Eng., 2018, 6(8): 10886-10895. |
| 24 | Niu Y X, Gao F, Zhu R M, et al. Solubility of dilute SO2 in mixtures of N,N-dimethylformamide + polyethylene glycol 400 and the density and viscosity of the mixtures[J]. J. Chem. Eng. Data, 2013, 58(3): 639-647. |
| 25 | Zhang J B, Han F, Zhang P Y, et al. Gas-liquid equilibrium data for mixture gas of sulfur dioxide + nitrogen with poly(ethylene glycol) aqueous solutions at 298.15 K and 122.61 kPa[J]. J. Chem. Eng. Data, 2010, 55(2): 959-961. |
| 26 | Li Q, Zhang J B, Li L H, et al. Solubility properties and spectral investigation of dilute SO2 in a triethylene glycol + water + La3+ system[J]. J. Phys. Chem. B, 2013, 117(18): 5633-5646. |
| 27 | Praumitz J M, Lichtenthaler R N, Azevedo E G. Molecular Thermodynamics of Fluid-Phase Equilibria [M]. 2nd ed. Prentice-Hall: Englewood Cliffs, NJ, 1986. |
| 28 | Augusta S. Determination of sulfur dioxide by ultraviolet absorption spectroscopy[J]. Anal. Chem., 1973, 45(9): 1744-1747. |
| 29 | Potteau E, Levillain E, Lelieur J P. Mechanism of the electrochemical reduction of sulfur dioxide in nonaqueous solvents[J]. J. Electroanal. Chem., 1999, 476(1): 15-25. |
| 30 | Wang C M, Cui G K, Luo X Y, et al. Highly efficient and reversible SO2 capture by tunable azole-based ionic liquids through multiple-site chemical absorption[J]. J. Am. Chem. Soc., 2011, 133(31): 11916-11919. |
| 31 | Yang Z Z, He L N, Zhao Y, et al. Highly efficient SO2 absorption and its subsequent utilization by weak base/polyethylene glycol binary system[J]. Environ. Sci. Technol., 2013, 47(3): 1598-1605. |
| 32 | Xu Q, Jiang W, Xiao J. Bet al. Absorption of sulfur dioxide by tetraglyme-sodium salt ionic liquid[J]. Molecules, 2019, 24(3): 436. |
| [1] | 杨欣, 王文, 徐凯, 马凡华. 高压氢气加注过程中温度特征仿真分析[J]. 化工学报, 2023, 74(S1): 280-286. |
| [2] | 黄琮琪, 吴一梅, 陈建业, 邵双全. 碱性电解水制氢装置热管理系统仿真研究[J]. 化工学报, 2023, 74(S1): 320-328. |
| [3] | 常明慧, 王林, 苑佳佳, 曹艺飞. 盐溶液蓄能型热泵循环特性研究[J]. 化工学报, 2023, 74(S1): 329-337. |
| [4] | 金正浩, 封立杰, 李舒宏. 氨水溶液交叉型再吸收式热泵的能量及 分析[J]. 化工学报, 2023, 74(S1): 53-63. 分析[J]. 化工学报, 2023, 74(S1): 53-63. |
| [5] | 张化福, 童莉葛, 张振涛, 杨俊玲, 王立, 张俊浩. 机械蒸汽压缩蒸发技术研究现状与发展趋势[J]. 化工学报, 2023, 74(S1): 8-24. |
| [6] | 米泽豪, 花儿. 基于DFT和COSMO-RS理论研究多元胺型离子液体吸收SO2气体[J]. 化工学报, 2023, 74(9): 3681-3696. |
| [7] | 胡建波, 刘洪超, 胡齐, 黄美英, 宋先雨, 赵双良. 有机笼跨细胞膜易位行为的分子动力学模拟研究[J]. 化工学报, 2023, 74(9): 3756-3765. |
| [8] | 张曼铮, 肖猛, 闫沛伟, 苗政, 徐进良, 纪献兵. 危废焚烧处理耦合有机朗肯循环系统工质筛选与热力学优化[J]. 化工学报, 2023, 74(8): 3502-3512. |
| [9] | 李锦潼, 邱顺, 孙文寿. 煤浆法烟气脱硫中草酸和紫外线强化煤砷浸出过程[J]. 化工学报, 2023, 74(8): 3522-3532. |
| [10] | 张瑞航, 曹潘, 杨锋, 李昆, 肖朋, 邓春, 刘蓓, 孙长宇, 陈光进. ZIF-8纳米流体天然气乙烷回收工艺的产品纯度关键影响因素分析[J]. 化工学报, 2023, 74(8): 3386-3393. |
| [11] | 胡兴枝, 张皓焱, 庄境坤, 范雨晴, 张开银, 向军. 嵌有超小CeO2纳米粒子的碳纳米纤维的制备及其吸波性能[J]. 化工学报, 2023, 74(8): 3584-3596. |
| [12] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [13] | 卫雪岩, 钱勇. 微米级铁粉燃料中低温氧化反应特性及其动力学研究[J]. 化工学报, 2023, 74(6): 2624-2638. |
| [14] | 陈宇豪, 陈晓平, 马吉亮, 梁财. 市政污泥回转窑焚烧气态污染物排放特性研究[J]. 化工学报, 2023, 74(5): 2170-2178. |
| [15] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号