化工学报 ›› 2020, Vol. 71 ›› Issue (6): 2840-2849.DOI: 10.11949/0438-1157.20200207
范小明1,2( ),陈希奎1,汪子涵1,曹帅1,程凤如1,杨则恒1,张卫新1(
),陈希奎1,汪子涵1,曹帅1,程凤如1,杨则恒1,张卫新1( )
)
收稿日期:2020-02-28
修回日期:2020-04-13
出版日期:2020-06-05
发布日期:2020-06-05
通讯作者:
张卫新
作者简介:范小明(1986—),男,博士,讲师,基金资助:
Xiaoming FAN1,2( ),Xikui CHEN1,Zihan WANG1,Shuai CAO1,Fengru CHENG1,Zeheng YANG1,Weixin ZHANG1(
),Xikui CHEN1,Zihan WANG1,Shuai CAO1,Fengru CHENG1,Zeheng YANG1,Weixin ZHANG1( )
)
Received:2020-02-28
Revised:2020-04-13
Online:2020-06-05
Published:2020-06-05
Contact:
Weixin ZHANG
摘要:
采用g-C3N4为自牺牲模板和氮源,葡萄糖为碳源,钼酸铵为钼源,制备具有二维纳米结构的氮掺杂碳化钼修饰碳纳米片(N-Mo2C/C),并评价其电催化析氢性能。利用X射线衍射仪(XRD)、场发射扫描电镜(FESEM)、透射电镜(TEM)、拉曼(Raman)等测试手段对N-Mo2C/C的组成、形貌及结构进行分析。结果表明,氮掺杂的Mo2C纳米颗粒均匀分散在二维碳纳米片上,粒径主要分布在3~5 nm。利用电化学工作站测试 N-Mo2C/C的电催化析氢性能,在1 mol/L KOH溶液中,电流密度为10 mA/cm2时其对应的过电势为185 mV,Tafel斜率为69 mV/dec,经20 h循环可维持稳定的析氢电势。
中图分类号:
范小明, 陈希奎, 汪子涵, 曹帅, 程凤如, 杨则恒, 张卫新. 自牺牲模板法制备氮掺杂碳化钼/碳析氢电催化剂[J]. 化工学报, 2020, 71(6): 2840-2849.
Xiaoming FAN, Xikui CHEN, Zihan WANG, Shuai CAO, Fengru CHENG, Zeheng YANG, Weixin ZHANG. Self-sacrificing templated preparation of nitrogen-doped molybdenum carbide/carbon as hydrogen evolution electrocatalyst[J]. CIESC Journal, 2020, 71(6): 2840-2849.

图3 NMC-700(a)、NMC-800(b)和NMC-900(c)的FESEM图;NMC-800的TEM图(d);NMC-800的HRTEM图(e)和选区电子衍射图(f);NMC-800TEM图(g)及相应的元素分布图(C,N和Mo元素)[(h)~(j)]
Fig.3 FESEM images of NMC-700 (a), NMC-800 (b) and NMC-900 (c); TEM image of NMC-800 (d) ; HRTEM image (e) and selected area electron diffraction pattern (f) of NMC-800; TEM image(g) and corresponding element mapping images of NMC-800 (C, N and Mo elements) [(h)~(j)]

图4 NMC-800样品的氮气吸附-脱附等温线(a)及对应的孔径分布曲线(b)
Fig.4 Nitrogen adsorption-desorption isotherm of NMC-800 (a), and corresponding pore size distribution curve (b)

图5 不同催化剂的C,N,Mo元素高分辨XPS谱图:NMC-700 (a)~(c),NMC-800 (d)~(f),NMC-900 (g)~(i)
Fig.5 High-resolution C, N, Mo XPS spectra of different catalysts: NMC-700 (a)—(c), NMC-800 (d)—(f), NMC-900 (g)—(i)
| 催化剂 | C/% (atom) | Mo/% (atom) | N/% (atom) | O/% (atom) | Mo-N/%(atom) |
|---|---|---|---|---|---|
| NMC-700 | 77.78 | 3.69 | 4.54 | 13.99 | 0.95 |
| NMC-800 | 75.68 | 5.25 | 8.66 | 10.41 | 2.27 |
| NMC-900 | 82.56 | 4.14 | 4.09 | 9.21 | 0.92 |
表1 高分辨XPS谱图中不同催化剂的各元素含量
Table 1 Contents of each element in different catalysts from high-resolution XPS spectra
| 催化剂 | C/% (atom) | Mo/% (atom) | N/% (atom) | O/% (atom) | Mo-N/%(atom) |
|---|---|---|---|---|---|
| NMC-700 | 77.78 | 3.69 | 4.54 | 13.99 | 0.95 |
| NMC-800 | 75.68 | 5.25 | 8.66 | 10.41 | 2.27 |
| NMC-900 | 82.56 | 4.14 | 4.09 | 9.21 | 0.92 |
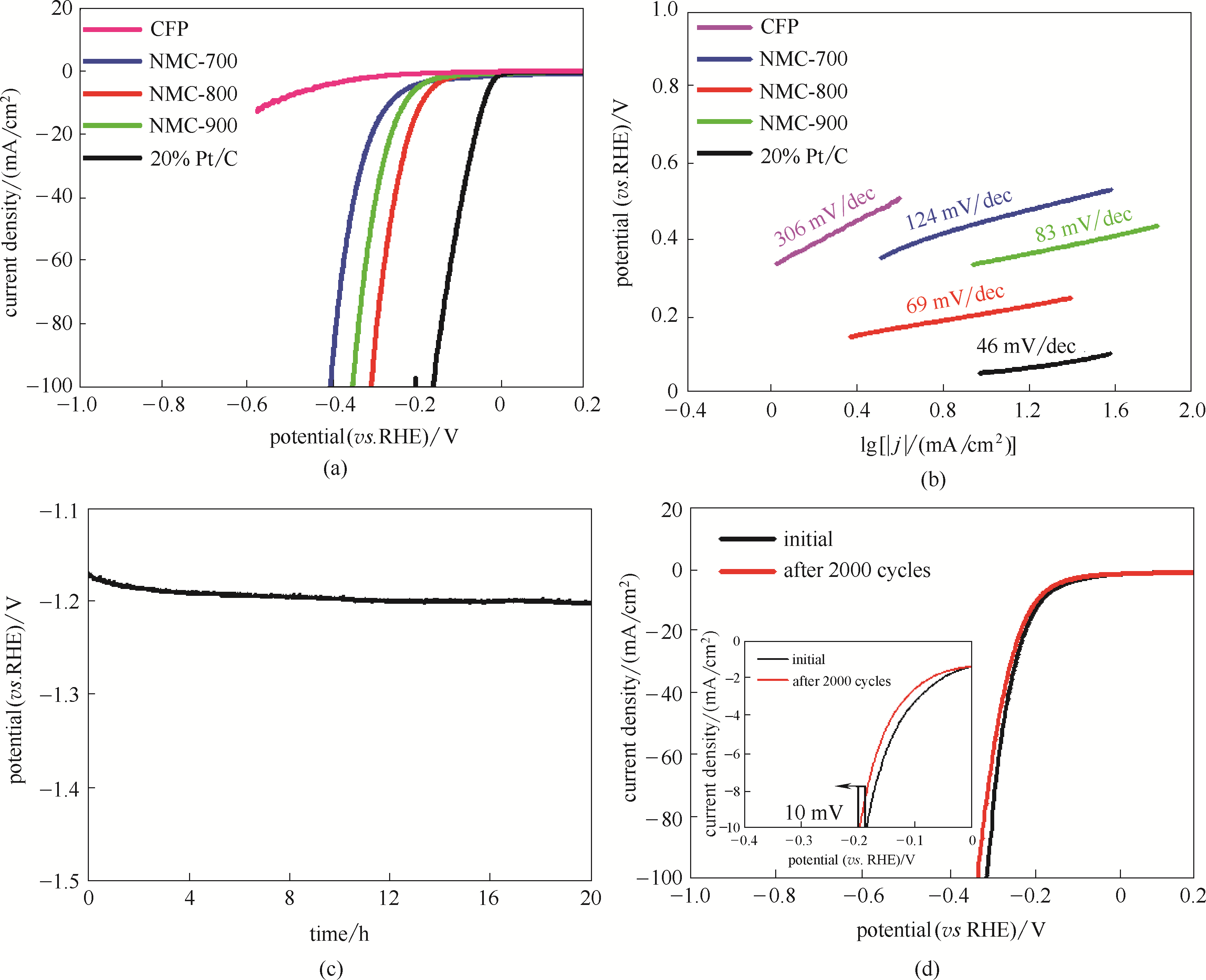
图6 CFP、NMC-700、NMC-800、NMC-900和商用Pt/C的极化曲线(a)和Tafel图(b)(电解液:1 mol/L KOH,扫描速率:10 mV/s);NMC-800在1 mol/L KOH中的稳定性测试曲线(c);NMC-800经过2000次循环前后的极化曲线(d)
Fig.6 Polarization curves (a) and Tafel plots of CFP, NMC-700, NMC-800, NMC-900 and commercial Pt/C (b) (scanning rate: 10 mV/s, electrolyte: 1 mol/L KOH); stability test curve of NMC-800 in 1 mol/L KOH (c); polarization curve of NMC-800 before and after 2000 cyclic voltammetry cycles (d)
| Electrocatalyst | j/ (mA/cm2) | η/(mV) | b/ (mV/dec ) | Electrolyte solution | Ref. |
|---|---|---|---|---|---|
| NMC-800 | 10 | 185 | 69 | 1M KOH | this work |
| MoC/C | 10 | ≈200 | 114 | 1M KOH | [ |
| Mo2C@N-C(S) | 10 | 271 | 90 | 1M KOH | [ |
| Mo2C NWAs/CFP | 10 | ≈168 | 72 | 1M KOH | [ |
| Mo/Mo2C@G-800 | 10 | 159 | 78 | 1M KOH | [ |
| Mo2C@NC | 10 | ≈247 | 78 | 1M KOH | [ |
表2 N-Mo2C/C催化剂的HER活性与文献报道结果比较
Table 2 Comparison of HER activity for N-Mo2C/C catalysts with the results in recently reported literatures
| Electrocatalyst | j/ (mA/cm2) | η/(mV) | b/ (mV/dec ) | Electrolyte solution | Ref. |
|---|---|---|---|---|---|
| NMC-800 | 10 | 185 | 69 | 1M KOH | this work |
| MoC/C | 10 | ≈200 | 114 | 1M KOH | [ |
| Mo2C@N-C(S) | 10 | 271 | 90 | 1M KOH | [ |
| Mo2C NWAs/CFP | 10 | ≈168 | 72 | 1M KOH | [ |
| Mo/Mo2C@G-800 | 10 | 159 | 78 | 1M KOH | [ |
| Mo2C@NC | 10 | ≈247 | 78 | 1M KOH | [ |

图7 NMC-700(a)、NMC-800(b)及NMC-900(c)在不同扫描速率下的CV曲线(1 mol/L KOH);NMC-700,NMC-800及NMC-900电容电流与扫描速率的关系(d)
Fig.7 CV curves of NMC-700 (a), NMC-800 (b) and NMC-900 (c) at different scanning rates (1 mol/L KOH); relationship between capacitor current and scanning rate for NMC-700, NMC-800 and NMC-900 (d)
| 1 | Kudo A, Miseki Y. Heterogeneous photocatalyst materials for water splitting[J]. Chem. Soc. Rev., 2009, 38(1): 253-278. |
| 2 | Pu Z, Amiinu I S, Kou Z, et al. RuP2-based catalysts with platinum-like activity and higher durability for hydrogen evolution reaction at all pH values[J]. Angew. Chem. Int. Ed., 2017, 56(38): 11559-11564. |
| 3 | Li Y, Chen W, Pei J, et al. Rational design of single molybdenum atoms anchored on N-doped carbon for effective hydrogen evolution reaction[J]. Angew. Chem. Int. Ed., 2017, 56(50): 16086-16090. |
| 4 | Pu Z, Xue Y, Amiinu I S, et al. Ultrasmall tungsten phosphide nanoparticles embedded in nitrogen-doped Carbon as a highly active and stable hydrogen-evolution electrocatalyst[J]. J. Mater. Chem. A., 2016, 4(40): 15327-15332. |
| 5 | Wang X, Wang H, Xu X, et al. 3D self-assembly of ultrafine molybdenum carbide confined in N-doped carbon nanosheets for efficient hydrogen production[J]. Nanoscale, 2017, 9(41): 15895-15900. |
| 6 | Lu Q, Yu Y, Ma Q, et al. 2D transition-metal-dichalcogenide-nanosheet-based composites for photocatalytic and electrocatalytic hydrogen evolution reactions[J]. Adv. Mater., 2016, 28(10): 1917-1933. |
| 7 | Xiao P, Ge X, Wang H, et al. Novel molybdenum carbide-tungsten carbide composite nanowires and their electrochemical activation for efficient and stable hydrogen evolution[J]. Adv. Funct. Mater., 2015, 25(10): 1520-1526. |
| 8 | Yuan W J, Huang Q, Yang X J, et al. Two-dimensional lamellar Mo2C for electrochemical hydrogen production: insights into the origin of hydrogen evolution reaction activity in acidic and alkaline electrolytes[J]. ACS Appl. Mater. Interfaces, 2018, 10(47): 40500-40508. |
| 9 | Xiao P, Sk M A, Thia L, et al. Molybdenum phosphide as an efficient electrocatalyst for the hydrogen evolution reaction[J]. Energy Environ. Sci., 2014, 7(8): 2624-2629. |
| 10 | Kimmel Y C, Xu X, Yu W, et al. Trends in electrochemical stability of transition metal carbides and their potential use as supports for low-cost electrocatalysts[J]. ACS Catal., 2014, 4(5): 1558-1562. |
| 11 | Gao Q, Zhang W, Shi Z, et al. Structural design and electronic modulation of transition‐metal‐carbide electrocatalysts toward efficient hydrogen evolution[J]. Adv. Mater., 2018, 31(2): 1802880. |
| 12 | Vrubel H, Hu X. Molybdenum boride and carbide catalyze hydrogen evolution in both acidic and basic solutions[J]. Angew. Chem. Int. Ed., 2012, 51(51): 12703-12706. |
| 28 | Ji L, Wang J, Teng X, et al. N, P-Doped molybdenum carbide nanofibers for efficient hydrogen production[J]. ACS Appl. Mater. Interfaces, 2018, 10(17): 14632-14640. |
| 29 | Tang C, Zhang H, Xu K, et al. Unconventional molybdenum carbide phases with high electrocatalytic activity for hydrogen evolution reaction[J]. J. Mater. Chem. A, 2019, 7(30): 18030-18038. |
| 13 | Wei H F, Xi Q Y, Chen X A, et al. Molybdenum carbide nanoparticles coated into the graphene wrapping N-doped porous carbon microspheres for highly efficient electrocatalytic hydrogen evolution both in acidic and alkaline media[J]. Adv. Sci., 2018, 5(3): 1700733. |
| 14 | Liu Z Q, Yang T O, Ye Y Q, et al. Heterostructures composed of N-doped carbon nanotubes encapsulating cobalt and β-Mo2C nanoparticles as bifunctional electrodes for water splitting [J]. Angew. Chem. Int. Ed., 2019, 58(15): 4923-4928. |
| 15 | Yu H J, Shang L, Bian T, et al. Carbon nanosheets: nitrogen-doped porous carbon nanosheets templated from g-C3N4 as metal-free electrocatalysts for efficient oxygen reduction reaction[J]. Adv. Mater., 2016, 28(25): 5140-5140. |
| 16 | Li B, Xi B, Feng Z, et al. Hierarchical porous nanosheets constructed by graphene-coated, interconnected TiO2 nanoparticles for ultrafast sodium storage[J]. Adv. Mater., 2018, 30(10): 1-9. |
| 17 | Chi J Q, Xie J Y, Zhang W W, et al. N‑Doped sandwich-structured Mo2C@C@Pt interface with ultralow Pt loading for pH-universal hydrogen evolution reaction[J]. ACS Appl. Mater. Interfaces, 2019, 11(4): 4047-4056. |
| 18 | Ji L, Wang J, Guo L, et al. In situ O2-emission assisted synthesis of molybdenum carbide nanomaterials as an efficient electrocatalyst for hydrogen production in both acidic and alkaline media[J]. J. Mater. Chem. A, 2017, 5(10): 5178-5186. |
| 30 | Han W W, Chen L L, Ma B, et al. Ultra-small Mo2C nanodots encapsulated in nitrogen doped porous carbon for pH-universal hydrogen evolution: insights into the synergetic enhancement by nitrogen doping and structure defects[J]. J. Mater. Chem. A, 2019, 7(42): 4734-4743. |
| 31 | Ji M, Niu S Q, Du Y C, et al. Anion induced size selection of β-Mo2C supported on nitrogen-doped carbon nanotubes for electrocatalytic hydrogen evolution[J]. ACS Sustain. Chem. Eng., 2018, 6(9): 11922-11929. |
| 32 | Zhang X, Zhou F, Pan W Y, et al. General construction of molybdenum-based nanowire arrays for pH-universal hydrogen evolution electrocatalysis[J]. Adv. Funct. Mater., 2018, 28(43): 1804600. |
| 33 | Zhu X Q, Zhang X Y, Huang B L, et al. An interfacial electron transfer relay center for accelerating the hydrogen evolution reaction[J]. J. Mater. Chem. A, 2019, 7(31): 18304-18310. |
| 19 | Zhang H B, Ma Z J, Duan J J, et al. Active sites implanted carbon cages in core-shell architecture: highly active and durable electrocatalyst for hydrogen evolution reaction[J]. ACS Nano., 2015, 10(1): 684-694. |
| 20 | Hou D, Zhu S Y, Tian H, et al. Two-dimensional sandwich-structured mesoporous Mo2C/carbon/graphene nanohybrids for efficient hydrogen production electrocatalysts[J]. ACS Appl. Mater. Interfaces, 2018, 10(47): 40800-40807. |
| 21 | 贺新福, 龙雪颖, 吴红菊, 等. 氮掺杂石墨烯/多孔碳复合材料的制备及其氧还原催化性能[J]. 化工学报, 2019, 70(6): 2308-2315. |
| He X F, Long X Y, Wu H J, et al. Preparation of nitrogen-doped graphene/porous carbon composite and oxygen reduction catalytic performance[J]. CIESC Journal, 2019, 70(6): 2308-2315. | |
| 22 | Jia J, Xiong T, Zhao L, et al. Ultrathin N-doped Mo2C nanosheets with exposed active sites as efficient electrocatalyst for hydrogen evolution reactions[J]. ACS Nano., 2017, 11(12): 12509-12518. |
| 23 | Wang J, Chen W, Wang T, et al. A strategy for highly dispersed Mo2C/MoN hybrid nitrogen-doped graphene via ion-exchange resin synthesis for efficient electrocatalytic hydrogen reduction[J]. Nano Res., 2018, 11(9): 4535-4548. |
| 24 | 水恒心, 潘冯弘康, 金田, 等. 双功能yolk-shell钴@钴氮碳掺杂氧电极催化剂[J]. 化工学报, 2018, 69(11): 4702-4712. |
| Shui H X, Panfeng H K, Jin T, et al. York-shell Co@Co-N/C of bifunctional oxygen electrocatalysts[J]. CIESC Journal, 2018, 69(11): 4702-4712. | |
| 25 | Lu C, Tranca D, Zhang J, et al. Molybdenum carbide-embedded nitrogen-doped porous carbon nanosheets as electrocatalysts for water splitting in alkaline media[J]. ACS Nano., 2017, 11(4): 3933-3942. |
| 26 | Liu B C, Li H, Cao B, et al. Few layered N, P dual-doped carbon-encapsulated ultrafine MoP nanocrystal/MoP cluster hybrids on carbon cloth: an ultrahigh active and durable 3D self-supported integrated electrode for hydrogen evolution reaction in a wide pH range[J]. Adv. Funct. Mater., 2018, 28(30): 1801527. |
| 34 | Xu Z X, Zhang G F, Lu C B, et al. Molybdenum carbide nanoparticles decorated hierarchical tubular carbon superstructures with vertical nanosheet arrays for efficient hydrogen evolution[J]. J. Mater. Chem. A, 2018, 6(39): 18833-18838. |
| 27 | Anjum M, Lee M H, Lee J S. Boron and nitrogen Co-doped molybdenum carbide nanoparticles imbedded in BCN network as a bifunctional electrocatalyst for hydrogen and oxygen evolution reactions[J]. ACS Catal., 2018, 8(9): 8296-8305. |
| [1] | 程业品, 胡达清, 徐奕莎, 刘华彦, 卢晗锋, 崔国凯. 离子液体基低共熔溶剂在转化CO2中的应用[J]. 化工学报, 2023, 74(9): 3640-3653. |
| [2] | 陈杰, 林永胜, 肖恺, 杨臣, 邱挺. 胆碱基碱性离子液体催化合成仲丁醇性能研究[J]. 化工学报, 2023, 74(9): 3716-3730. |
| [3] | 李艺彤, 郭航, 陈浩, 叶芳. 催化剂非均匀分布的质子交换膜燃料电池操作条件研究[J]. 化工学报, 2023, 74(9): 3831-3840. |
| [4] | 刘远超, 关斌, 钟建斌, 徐一帆, 蒋旭浩, 李耑. 单层XSe2(X=Zr/Hf)的热电输运特性研究[J]. 化工学报, 2023, 74(9): 3968-3978. |
| [5] | 杨学金, 杨金涛, 宁平, 王访, 宋晓双, 贾丽娟, 冯嘉予. 剧毒气体PH3的干法净化技术研究进展[J]. 化工学报, 2023, 74(9): 3742-3755. |
| [6] | 胡亚丽, 胡军勇, 马素霞, 孙禹坤, 谭学诣, 黄佳欣, 杨奉源. 逆电渗析热机新型工质开发及电化学特性研究[J]. 化工学报, 2023, 74(8): 3513-3521. |
| [7] | 陈佳起, 赵万玉, 姚睿充, 侯道林, 董社英. 开心果壳基碳点的合成及其对Q235碳钢的缓蚀行为研究[J]. 化工学报, 2023, 74(8): 3446-3456. |
| [8] | 杨欣, 彭啸, 薛凯茹, 苏梦威, 吴燕. 分子印迹-TiO2光电催化降解增溶PHE废水性能研究[J]. 化工学报, 2023, 74(8): 3564-3571. |
| [9] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [10] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [11] | 张蒙蒙, 颜冬, 沈永峰, 李文翠. 电解液类型对双离子电池阴阳离子储存行为的影响[J]. 化工学报, 2023, 74(7): 3116-3126. |
| [12] | 邢美波, 张中天, 景栋梁, 张洪发. 磁调控水基碳纳米管协同多孔材料强化相变储/释能特性[J]. 化工学报, 2023, 74(7): 3093-3102. |
| [13] | 余娅洁, 李静茹, 周树锋, 李清彪, 詹国武. 基于天然生物模板构建纳米材料及集成催化剂研究进展[J]. 化工学报, 2023, 74(7): 2735-2752. |
| [14] | 葛加丽, 管图祥, 邱新民, 吴健, 沈丽明, 暴宁钟. 垂直多孔碳包覆的FeF3正极的构筑及储锂性能研究[J]. 化工学报, 2023, 74(7): 3058-3067. |
| [15] | 屈园浩, 邓文义, 谢晓丹, 苏亚欣. 活性炭/石墨辅助污泥电渗脱水研究[J]. 化工学报, 2023, 74(7): 3038-3050. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号