化工学报 ›› 2020, Vol. 71 ›› Issue (3): 1234-1245.DOI: 10.11949/0438-1157.20191532
赵文英1,2,李文文2,孙晓岩2,曹晓荣1,项曙光1,2( )
)
收稿日期:2019-12-18
修回日期:2020-01-06
出版日期:2020-03-05
发布日期:2020-03-05
通讯作者:
项曙光
基金资助:
Wenying ZHAO1,2,Wenwen LI2,Xiaoyan SUN2,Xiaorong CAO1,Shuguang XIANG1,2( )
)
Received:2019-12-18
Revised:2020-01-06
Online:2020-03-05
Published:2020-03-05
Contact:
Shuguang XIANG
摘要:
用11类70种非极性、弱极性和极性物质的实验蒸汽压数据对适用于Peng-Robinson状态方程(PR EoS)的17种普遍化温度函数对蒸汽压的预测能力进行了评价。根据各类物质蒸汽压预测结果平均相对偏差评价普遍化温度函数的预测能力。结果表明,Robinson-Peng (1978)、汪萍(2004)和Li-Yang (2011)温度函数能够准确预测烷烃、芳烃、气体和卤代烃类物质的蒸汽压,但对弱极性和极性的醇、醚、酯、酸、水等物质的预测结果不如Forero (2016)温度函数准确。以偏心因子和极性因子普遍化的温度函数对醇、酸、水等极性物质蒸汽压的预测结果明显优于仅以偏心因子普遍化的温度函数。
中图分类号:
赵文英, 李文文, 孙晓岩, 曹晓荣, 项曙光. 基于PR立方型状态方程普遍化温度函数的研究与评价[J]. 化工学报, 2020, 71(3): 1234-1245.
Wenying ZHAO, Wenwen LI, Xiaoyan SUN, Xiaorong CAO, Shuguang XIANG. Research and evaluation on generalized alpha functions based on PR EoS[J]. CIESC Journal, 2020, 71(3): 1234-1245.
| 函数名称 | 参考文献 | 温度函数及参数关联式 |
|---|---|---|
Peng-Robinson (1976) | [ | |
Robinson-Peng (1978) | [ | |
Gasem (2001) | [ | |
| Coquelet (2004) | [ | |
| 汪萍(2004) | [ | |
| Joshipura (2009) | [ | |
| Li-Yang (2011) | [ | l= |
| Haghtalab (2011) | [ | |
| Saffari-Zahedi (2013) | [ | |
| Hou (2015) | [ | |
| Valiollahi (2016) | [ | |
| Le Guennec (2016) | [ | |
| Forero (2016) | [ | |
| Twu-Mahmoodi (2017) | [ | |
| Coquelet-Mahmoodi (2017) | [ | |
| PR-PM (2017) | [ | |
| PR-PM2 (2017) | [ |
表1 用于评价的普遍化温度函数
Table 1 Generalized alpha functions for evaluation
| 函数名称 | 参考文献 | 温度函数及参数关联式 |
|---|---|---|
Peng-Robinson (1976) | [ | |
Robinson-Peng (1978) | [ | |
Gasem (2001) | [ | |
| Coquelet (2004) | [ | |
| 汪萍(2004) | [ | |
| Joshipura (2009) | [ | |
| Li-Yang (2011) | [ | l= |
| Haghtalab (2011) | [ | |
| Saffari-Zahedi (2013) | [ | |
| Hou (2015) | [ | |
| Valiollahi (2016) | [ | |
| Le Guennec (2016) | [ | |
| Forero (2016) | [ | |
| Twu-Mahmoodi (2017) | [ | |
| Coquelet-Mahmoodi (2017) | [ | |
| PR-PM (2017) | [ | |
| PR-PM2 (2017) | [ |
| 物质类别 | 中文名称 | 数据点数 | 蒸汽压数据参考文献 |
|---|---|---|---|
| 正构烷烃 | C1~C20、C22、C24 | 1434 | [ |
| 正构醇 | C1~C10、C12、C14、C16、C18 | 826 | [ |
| 芳烃 | 苯、甲苯、乙苯、二甲苯(邻、间、对)、萘、1-甲基萘 | 559 | [ |
| 卤代烃 | 1,1-二氯-1-氟乙烷(R-141b) | 75 | [ |
| 气体 | 氮气、氩、氪、氙、一氧化碳、氨 | 404 | [ |
| 酸 | 乙酸、丙酸 | 66 | [ |
| 醚 | 二甲醚、二丙醚、乙丙醚、甲基正丁基醚 | 142 | [ |
| 酮 | 丙酮 | 47 | [ |
| 酯 | 甲酸甲酯、乙酸甲酯、丙酸甲酯、丁酸甲酯、异丁酸甲酯、甲酸乙酯、乙酸乙酯、丙酸乙酯、甲酸丙酯、乙酸丙酯 | 645 | [ |
| 杂环 | 1,4-二 烷 烷 | 66 | [ |
| 水 | 水 | 189 | [ |
表2 11类物质基本信息
Table 2 Basic information of 11 kinds of compounds
| 物质类别 | 中文名称 | 数据点数 | 蒸汽压数据参考文献 |
|---|---|---|---|
| 正构烷烃 | C1~C20、C22、C24 | 1434 | [ |
| 正构醇 | C1~C10、C12、C14、C16、C18 | 826 | [ |
| 芳烃 | 苯、甲苯、乙苯、二甲苯(邻、间、对)、萘、1-甲基萘 | 559 | [ |
| 卤代烃 | 1,1-二氯-1-氟乙烷(R-141b) | 75 | [ |
| 气体 | 氮气、氩、氪、氙、一氧化碳、氨 | 404 | [ |
| 酸 | 乙酸、丙酸 | 66 | [ |
| 醚 | 二甲醚、二丙醚、乙丙醚、甲基正丁基醚 | 142 | [ |
| 酮 | 丙酮 | 47 | [ |
| 酯 | 甲酸甲酯、乙酸甲酯、丙酸甲酯、丁酸甲酯、异丁酸甲酯、甲酸乙酯、乙酸乙酯、丙酸乙酯、甲酸丙酯、乙酸丙酯 | 645 | [ |
| 杂环 | 1,4-二 烷 烷 | 66 | [ |
| 水 | 水 | 189 | [ |
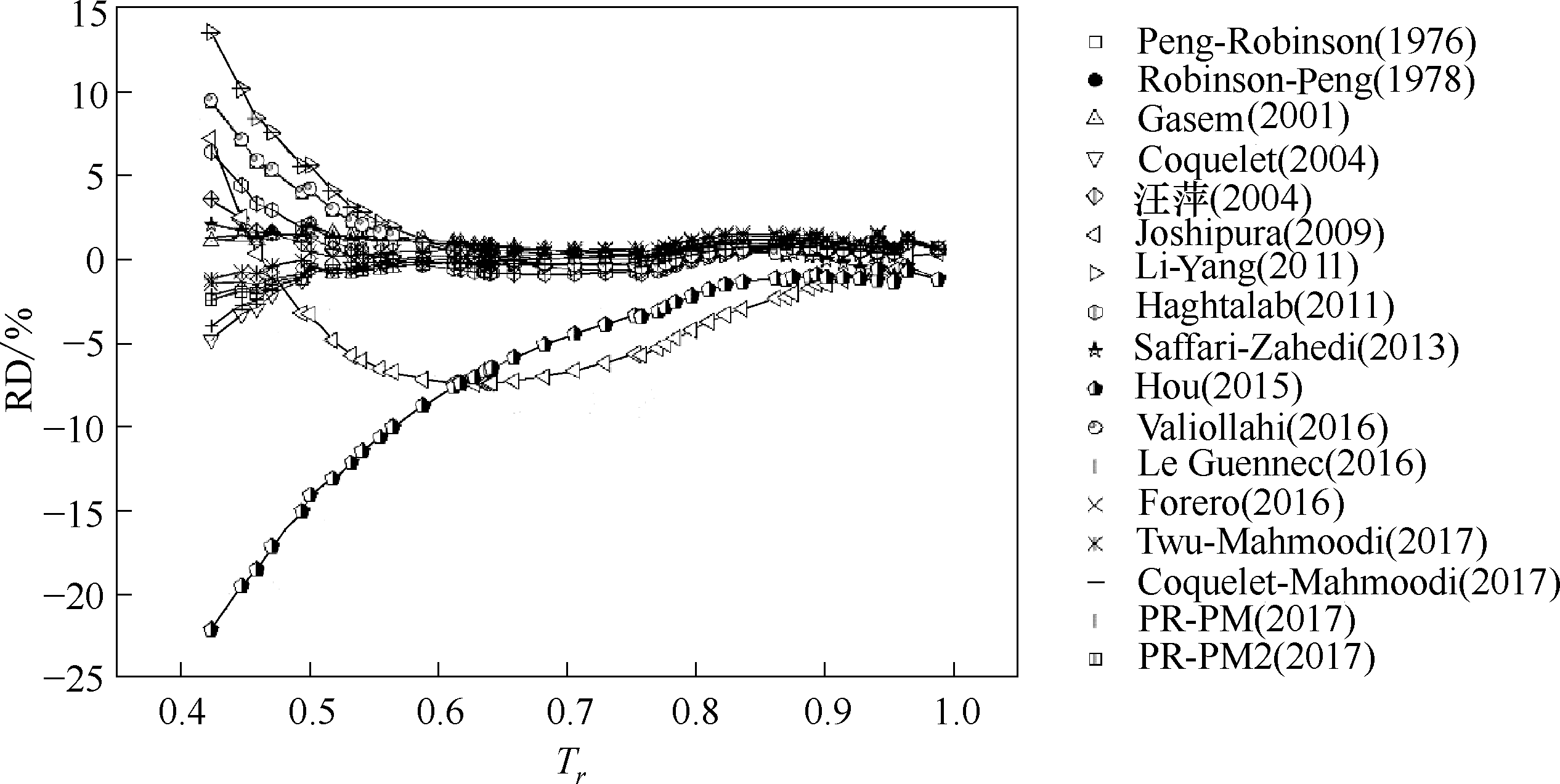
图3 参数普遍化温度函数对正丁烷蒸汽压预测结果的相对偏差随对比温度变化趋势
Fig.3 RDs of generalized alpha functions on prediction of vapor pressures ofn-butane with increasing reduced temperature
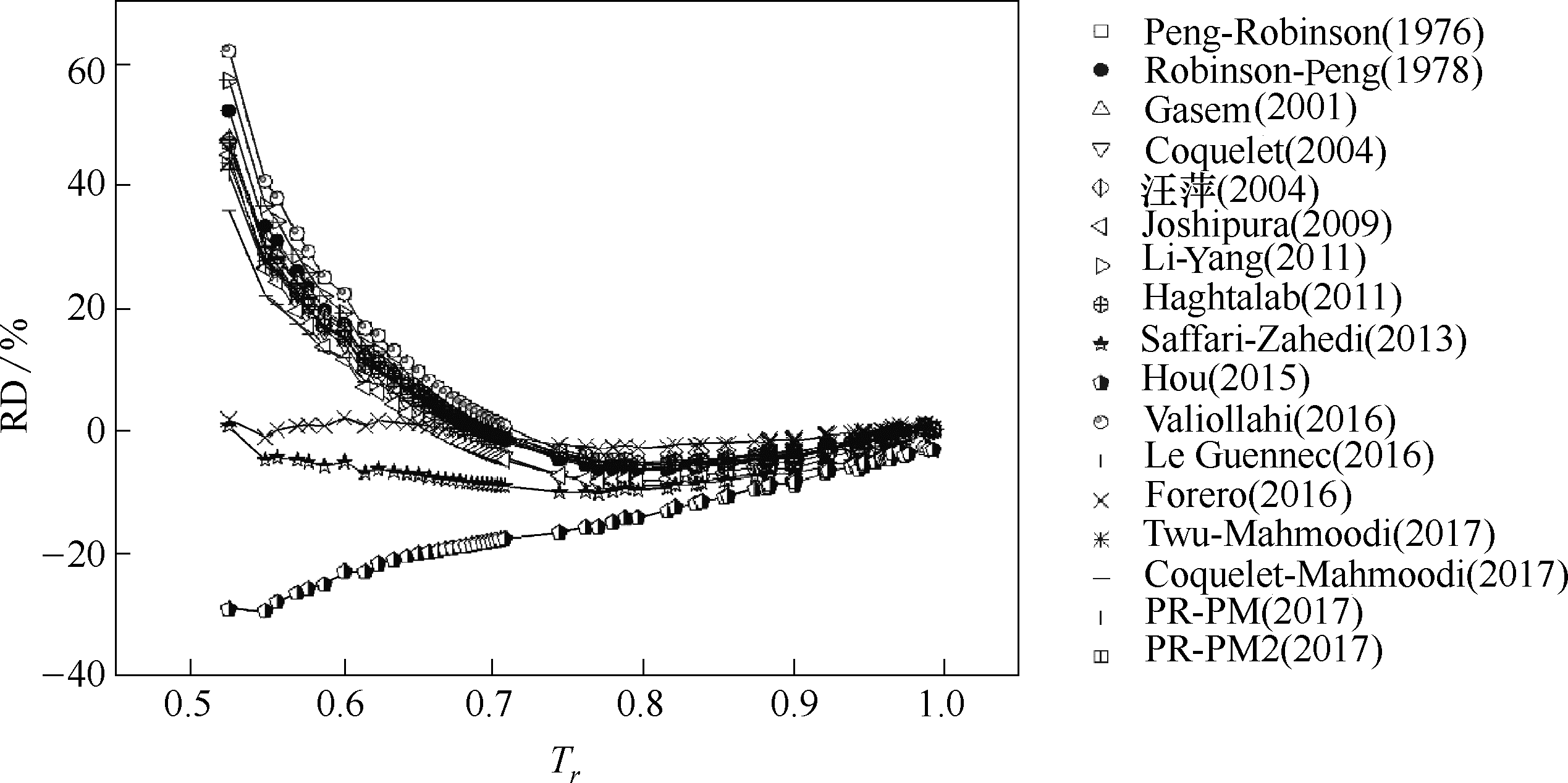
图5 参数普遍化温度函数对正丁醇蒸汽压预测结果的相对偏差随对比温度的变化趋势
Fig.5 RDs of generalized alpha functions on prediction of vapor pressures ofn-butanol with increasing reduced temperature
| 1 | van der Waals J H.On the continuity of the gaseous and liquid state [D].Holland:Leiden University,1873. |
| 2 | Redlich O,Kwong J N S.On the thermodynamics of solutions(V): An equation of state. Fugacities of gaseous solutions [J].Chem. Rev.,1949,44(1):233-244. |
| 3 | Soave G.Equilibrium constants from a modified Redlich-Kwong equation of state [J].Chem. Eng. Sci.,1972,27(6):1197-1203. |
| 4 | Peng D Y,Robinson D B.A new two-constant equation of state [J].Ind. Eng. Chem. Fundam.,1976,15(1):59-64. |
| 5 | Mathias P M,Copeman T W.Extension of the Peng-Robinson equation of state to complex mixtures: evaluation of the various forms of the local composition concept [J].Fluid Phase Equilibr.,1983,13:91-108. |
| 6 | Stryjek R,Vera J H.An improved cubic equation of state.Equations of state [C]//ACS Symp.Ser.,1986:560-570. |
| 7 | Twu C H.A modified Redlich-Kwong equation of state for highly polar,supercritical systems [C]//Proc.Int.Symp. Thermodyn. Chem. Eng. Ind.,1988:148-169. |
| 8 | Twu C H,Bluck D,Cunningham J R,et al.A cubic equation of state with a new alpha function and a new mixing rule [J].Fluid Phase Equilibr.,1991,69(91):33-50. |
| 9 | Gasem K A M,Gao W,Pan Z,et al.A modified temperature dependence for the Peng-Robinson equation of state [J].Fluid Phase Equilibr.,2001,181(1):113-125. |
| 10 | Le Guennec Y,Privat R,Jaubert J N.Development of the translated-consistent tc-PR and tc-RK cubic equations of state for a safe and accurate prediction of volumetric, energetic and saturation properties of pure compounds in the sub- and super-critical domains [J].Fluid Phase Equilibr.,2016,429:301-312. |
| 11 | Mahmoodi P,Sedigh M.A consistent and precise alpha function for cubic equations of state [J].Fluid Phase Equilibr.,2017,436:69-84. |
| 12 | Pina-Martinez A,Le Guennec Y,Privat R,et al.Analysis of the combinations of property data that are suitable for a safe estimation of consistent twu α-function parameters: updated parameter values for the translated-consistent tc-PR and tc-RK cubic equations of state [J].J. Chem. Eng. Data,2018,63(10):3980-3988. |
| 13 | Ghoderao P N P,Dalvi V H,Narayan M.A five-parameter cubic equation of state for pure fluids and mixtures [J].Chem. Eng. Sci.: X,2019,3:100026. |
| 14 | Zhao W,Sun X,Xia L,et al.Research into the polynomial alpha function for the cubic equation of state [J].Ind. Eng. Chem. Res.,2018,57(38):12602-12623. |
| 15 | Twu C H,Coon J E,Cunningham J R.An approach for the extension of a 3-parameter cubic equation of state to heavy hydrocarbons [J].Fluid Phase Equilibr.,1995,104:83-96. |
| 16 | Coquelet C,Chapoy A,Richon D.Development of a new alpha function for the Peng-Robinson equation of state: comparative study of alpha function models for pure gases (natural gas components) and water-gas systems [J].Int. J. Thermophys.,2004,25(1):133-158. |
| 17 | 汪萍,李忠杰,项曙光.低对比温度下PRSV方程的新温度函数关联式[J].石油化工,2004,33(10):951-955. |
| Wang P,Li Z J,Xiang S G.A new alpha function of PRSV equation at low relative temperatures [J].Petrochem. Tech.,2004,33(10):951-955. | |
| 18 | Li H,Yang D.Modified α function for the Peng-Robinson equation of state to improve the vapor pressure prediction of non-hydrocarbon and hydrocarbon compounds [J].Energ. Fuel.,2011,25(1):1-4. |
| 19 | Forero L A,Velasquez J A.A generalized cubic equation of state for non-polar and polar substances [J].Fluid Phase Equilibr.,2016,418:74-87. |
| 20 | Robinson D B,Peng D Y.The Characterization of the Heptanes and Heavier Fractions for the GPA Peng-Robinson Programs [M].US:Gas Processors Association,1978. |
| 21 | Valiollahi S,Kavianpour B,Raeissi S,et al.A new Peng-Robinson modification to enhance dew point estimations of natural gases [J]. J. Nat. Gas Sci. Eng.,2016,34:1137-1147. |
| 22 | Joshipura M H,Dabke S P,Subrahmanyam N.Development and comparison of cohesion function relationship for PR equation of state [J].Int. J. Chem. Eng. Res.,2009,1(2):123-134. |
| 23 | Haghtalab A,Mahmoodi P,Mazloumi S H.A modified Peng-Robinson equation of state for phase equilibrium calculation of liquefied, synthetic natural gas, and gas condensate mixtures [J].Can. J. Chem. Eng.,2011,89(6):1376-1387. |
| 24 | Saffari H,Zahedi A.A new alpha-function for the Peng-Robinson equation of state application to natural gas [J].Chinese J. Chem. Eng.,2013,21(10):1155-1161. |
| 25 | Hou D,Deng H,Zhang H,et al.Phase behavior and physical parameters of natural gas mixture with CO2 [J].J. Chem.,2015,2015:1-11. |
| 26 | Heyen G.A cubic equation of state with extended range of application[C]//Proceeding of the 2nd World Congress of Chemical Engineering.Montreal, Canada,1981. |
| 27 | Yaws C L.Thermophysical Properties of Chemicals and Hydrocarbons [M].Holland:Elsevier Science,2008. |
| 28 | Salerno S,Cascella M,May D,et al.Prediction of vapor pressures and saturated volumes with a simple cubic equation of state(I): A reliable data base [J].Fluid Phase Equilibr.,1986,27(86):15-34. |
| 29 | Sage B H,Lacey W N.Phase equilibria in hydrocarbon systems-thermodynamic properties of pentane [J].Ind. Eng. Chem.,1942,34:730-737. |
| 30 | Straty G C,Tsumura R.PVT and vapor pressure measurements on ethane [J]. J. Res. Nat. Bur. Stand.,1976,80A(1):35-39. |
| 31 | Sasse K,Jose J,Merlin J C.A static apparatus for measurement of low vapor pressures. Experimental results on high molecular-weight hydrocarbons [J].Fluid Phase Equilibr.,1988,42(8):287-304. |
| 32 | Wolff H,Shadiakhy A.The vapor pressure behavior and association of mixtures of 1-hexanol andn-hexane between 293 and 373 K [J].Fluid Phase Equilibr.,1981,7(3/4):309-325. |
| 33 | Bich E,Lober T,Millat J.Quasi-isochoric PPT measurements, 2nd virial coefficient and vapor pressure ofn-hexane [J].Fluid Phase Equilibr.,1992,75:149-161. |
| 34 | Ewing M B,Sanchez Ochoa J C.Vapour pressures ofn-hexane determined by comparative ebulliometry [J].J. Chem. Thermophys.,2006,38(3):283-288. |
| 35 | Dahmani A,Ait Kaci A,Jose J.Vapor pressures and excess functions of 1,4-dimethylpiperazine +n-heptane, or cyclohexane measurement and prediction [J].Fluid Phase Equilibr.,1997,134(1/2):255-265. |
| 36 | Forziati A F,Norris W R,Rossini F D.Vapor pressures and boiling points of sixty API-NBS hydrocarbons [J].J. Res. Nat. Bur. Stand.,1949,43:555-563. |
| 37 | Reddy P,Raal J D,Ramjugernath D.A novel dynamic recirculating apparatus for vapour-liquid equilibrium measurements at moderate pressures and temperatures [J].Fluid Phase Equilibr.,2013,358:121-130. |
| 38 | Weber L A.Vapor pressure of heptane from the triple point to the critical point [J].J. Chem. Eng. Data,2000,45(2):173-176. |
| 39 | Ewing M B,Sanchez Ochoa J C.Vapor pressures ofn-heptane determined by comparative ebulliometry [J].J. Chem. Eng. Data,2005,50(5):1543-1547. |
| 40 | Ewing M B,Sanchez Ochoa J C.The vapour pressures ofn-octane determined using comparative ebulliometry [J].Fluid Phase Equilibr.,2003,210(2):277-285. |
| 41 | Gregorowicz J,Kiciak K,Malanowski S.Vapor pressure data for 1-butanol, cumene,n-octane andn-decane and their statistically consistent reduction with the antoine equation [J].Fluid Phase Equilibr.,1987,38(1/2):97-107. |
| 42 | Allemand N,Jose J,Merlin J C.Vapor pressures of hydrocarbons (C10-C18 alkanes and alkylbenzenes) at 3-1000 Pa [J].Thermochim. Acta,1986,105:79-90. |
| 43 | Morgan D L,Kobayashi R.Direct vapor pressure measurements of tenn-alkanes in the C10-C28 range [J].Fluid Phase Equilibr.,1994,97(1/2):211-242. |
| 44 | Lee C H,Dempsey D M,Mohamed R S,et al.Vapor-liquid equilibria in the systems ofn-decane/tetralin,n-hexadecane/tetralin,n-decane/1-methylnaphthalene, and 1-methylnaphthalene/tetralin [J].J. Chem. Eng. Data,1992,37(2):183-186. |
| 45 | 马沛生.石油化工基础数据手册(续编)[M].北京:化学工业出版社,1993. |
| Ma P S.Handbook of Petrochemical Basic Data (Sequel Edition) [M].Beijing:Chemical Industry Press,1993. | |
| 46 |
Ambrose D,Sprake C H S.Thermodynamic properties of organic oxygen compounds( ):Vapor pressures and normal boiling temperatures of aliphatic alcohols [J].J. Chem. Thermodyn.,1970,2(5):631-645. ):Vapor pressures and normal boiling temperatures of aliphatic alcohols [J].J. Chem. Thermodyn.,1970,2(5):631-645.
|
| 47 | Nasirzadeh K,Neueder R,Kunz W.Vapor pressure determination of the aliphatic C5 to C8 1-alcohols [J].J. Chem. Eng. Data,2006,51(1):7-10. |
| 48 | Munday E B,Mullins J C,Edie D D.Vapor pressure data for toluene, 1-pentanol, 1-butanol, water, and 1-propanol and for the water and 1-propanol system from 273.15 to 323.15 [J].J. Chem. Eng. Data,1980,25(3):191-194. |
| 49 | Kemme H R,Kreps S I.Vapor pressure of primaryn-alkyl chlorides and alcohols [J].J. Chem. Eng. Data,1969,14(1):98-102. |
| 50 | Efremov Y V.Density, surface tension, saturated vapor pressures, and critical parameters of alcohols [J].Russ. J. Phys. Chem. A,1966,40(6):1240-1247. |
| 51 | Ambrose D,Townsend R.Thermodynamic properties of organic oxygen compounds(Ⅸ): The critical properties and vapor pressures, above five atmospheres, of six aliphatic alcohols [J].J. Chem. Soc.,1963,681:3614-3625. |
| 52 | Ambrose D,Sprake C H S,Townsend R.Thermodynamic properties of organic oxygen compounds(ⅩⅩⅩⅦ): Vapor pressures of methanol, ethanol, pentan-1-ol, and octan-1-ol from the normal boiling temperature to the critical temperature [J].J. Chem. Thermodyn.,1975,7(2):185-190. |
| 53 | Roganov G N,Pisarev P N,Emel'yanenko V N,et al.Measurement and prediction of thermochemical properties. Improved benson-type increments for the estimation of enthalpies of vaporization and standard enthalpies of formation of aliphatic alcohols [J].J. Chem. Eng. Data,2005,50(4):1114-1124. |
| 54 | Censky M,Rohac V,Ruzicka K,et al.Vapor pressure of selected aliphatic alcohols by ebulliometry. Part 1 [J].Fluid Phase Equilibr,2010,298(2):192-198. |
| 55 | N'guimbi J,Kasehgari H,Mokbel I,et al.Vapor pressure of primary alcohols at 0.3 Pa to 1.5 kPa [J].Thermochim. Acta,1992,196(2):367-377. |
| 56 | Ambrose D,Ellender J H,Sprake C H S.Thermodynamic properties of organic oxygen compounds(ⅩⅩⅩⅤ):Vapor pressures of aliphatic alcohols[J].J. Chem. Thermodyn.,1974,6(9):909-914. |
| 57 | Naziev Y M,Shakhverdiev A N,Akhundov T S,et al.Thermal properties of undecyl and dodecyl alcohols [J].Izv. Vyssh. Uchebn. Zaved.,1990, (12):69-72. |
| 58 | Fiock E F,Ginning D C,Holton W B.Calorimetric determinations of thermal properties of methyl alcohol, ehtyl alcohol, and benzene [J].Bur. Stand. J. Res.,1931,6:881-900. |
| 59 | Butcher K L,Ramasubramanian K R,Medani M S.Thermodynamic properties of the benzene andn-heptane system at elevated temperatures [J].J. App. Chem. Biotech.,1972,22(11):1139-1155. |
| 60 | Ambrose D.Vapor pressures of some aromatic hydrocarbons [J].J. Chem. Thermodyn.,1987,19(9):1007-1008. |
| 61 | Kalafati D D,Rasskazov D S,Petrov E K.Determination of the temperature dependence of the saturation pressure of benzene [J].Zh. Fiz. Khim.,1967,41(6):1357-1359. |
| 62 | de Kruif C G,Kuipers T,van Miltenburg J C,et al.The vapor pressure of solid and liquid naphthalene [J].J. Chem. Thermodyn.,1981,13(11):1081-1086. |
| 63 | Chirico R D,Knipmeyer S E,Nguyen A,et al.The thermodynamic properties to the temperature 700 K of naphthalene and of 2,7-dimethylnaphthalene [J].J. Chem. Thermodyn.,1993,25(12):1461-1494. |
| 64 | Schroer E.Critical state(Ⅵ): Vapor-pressure curve of naphthalene up to the critical point [J]. Z. Physik. Chem.,1941, B49:271-278. |
| 65 | Wieczorek S A,Kobayashi R.Vapor-pressure measurements of 1-methylnaphthalene, 2-methylnaphthalene, and 9,10-dihydrophenanthrene at elevated temperatures[J].J. Chem. Eng. Data,1981,26(1):8-11. |
| 66 | Chirico R D,Knipmeyer S E,Nguyen A,et al.Thermodynamic equilibria in xylene isomerization(4): The thermodynamic properties of ethylbenzene [J].J. Chem. Eng. Data,1997,42(4):772-783. |
| 67 | Vonniederhausern D M,Wilson G M,Giles N F.Critical point and vapor pressure measurements at high temperatures by means of a new apparatus with ultralow residence times [J].J. Chem. Eng. Data,2000,45(2):157-160. |
| 68 | Pitzer K S,Scott D W.The thermodynamics and molecular structure of benzene and its methyl derivatives[J].J. Am. Chem. Soc.,1943,65:803-829. |
| 69 | Hugill J A,Mcglashan M L.The vapor pressure from 451 K to the critical temperature, and the critical temperature and critical pressure, of cyclohexane [J].J. Chem. Thermodyn.,1978,10(1):95-100. |
| 70 | Mokbel I,Rauzy E,Meille J P,et al.Low vapor pressures of 12 aromatic hydrocarbons. Experimental and calculated data using a group contribution method [J].Fluid Phase Equilibr.,1998,147(1/2):271-284. |
| 71 | Lee C H,Holder G D.Vapor-liquid equilibria in the systems toluene/naphthalene and cyclohexane/naphthalene [J].J. Chem. Eng. Data,1993,38(2):320-323. |
| 72 | Watanabe N,Yokoyama C,Takahashi S.PVT relationship of gaseous toluene at temperatures from 500 K to 600 K [J].Kagaku. Kogaku. Ronbunshu.,1988,14(4):525-530. |
| 73 | Duarte-Garza H A,Hwang C A,Kellerman S A,et al.Vapor pressure, vapor density, and liquid density for 1,1-dichloro-1-fluoroethane (R-141b) [J].J. Chem. Eng. Data,1997,42(3):497-501. |
| 74 | Defibaugh D R,Goodwin A R H,Morrison G,et al.Thermodynamic properties of 1,1-dichloro-1-fluoroethane (R141b) [J].Fluid Phase Equilibr.,1993,85:271-284. |
| 75 | Zhao Y,Dong X,Gong M,et al.Apparatus for low-temperature investigations: phase equilibrium measurements for systems containing ammonia [J].J. Chem. Eng. Data,2016,61(11):3883-3889. |
| 76 | Sato M,Masui G,Uematsu M.Critical parameters for ammonia [J].J. Chem. Thermodyn.,2005,37(9):931-934. |
| 77 | Kasahara K,Munakata T,Uematsu M.(P,ρ,T) measurements of liquid ammonia in the temperature range fromT = 310 K toT = 400 K at pressures up toP = 17 MPa [J].J. Chem. Thermodyn.,1999,31(10):1273-1281. |
| 78 | Streatfeild M H,Henderson C,Staveley L A K,et al.Some thermodynamic properties of liquid ammonia and trideuteroammonia [J].J. Chem. Thermodyn.,1987,19(11):1163-1171. |
| 79 | Holcomb C D,Outcalt S L.Near-saturation (P, ρ, T) and vapor-pressure measurements of NH3, and liquid-phase isothermal (P, ρ, T) and bubble-point-pressure measurements of NH3 + H2O mixtures [J].Fluid Phase Equilibr.,1999,164(1):97-106. |
| 80 | Wagner W.New vapor pressure measurements for argon and nitrogen and a new method for establishing rational vapor pressure equations [J].Cryogenics,1973,13(8):470-482. |
| 81 | Bowman D H,Aziz R A,Lim C C.Vapor pressure of liquid argon, krypton, and xenon [J].Can. J. Phys.,1969,47(3):267-273. |
| 82 | Shinoda T.Vapor pressure of carbon monoxide in condensed phases [J].Bullchemsocjpn,1969,42(10):2815-2820. |
| 83 | Theeuwes F,Bearman R J.P,V,T behavior of dense fluids(Ⅴ): Vapor pressure and saturated liquid density of xenon [J].J. Chem. Thermodyn.,1970,2(4):507-512. |
| 84 | Crommelin C A,Bijleveld W J,Brown E G.Vapor tensions, critical point and triple point of carbon monoxide [J].Proc. K. Ned. Akad. Wet.,1931,34:1314-1317. |
| 85 | Potter A E,Ritter H L.The vapor pressure of acetic acid and acetic-d3 acid-d. The liquid density of acetic-d3 acid-d [J].J. Phys. Chem.,1954,58:1040-1042. |
| 86 | Ambrose D,Ellender J H,Sprake C H S,et al.Thermodynamic properties of organic oxygen compounds(ⅩLⅤ): The vapor pressure of acetic acid [J].J. Chem. Thermodyn.,1977,9(8):735-741. |
| 87 | Ambrose D,Ellender J H,Gundry H A,et al.Thermodynamic properties of organic oxygen compounds. LI. The vapor pressures of some esters and fatty acids [J].J. Chem. Thermodyn.,1981,13(8):795-802. |
| 88 | Wu J,Yin J.Vapor pressure measurements of dimethyl ether from (213 to 393) K [J].J. Chem. Eng. Data,2008,53(9):2247-2249. |
| 89 | Tanaka K,Higashi Y.Measurements of the isobaric specific heat capacity and density for dimethyl ether in the liquid state [J].J. Chem. Eng. Data,2010,55(8):2658-2661. |
| 90 | Ambrose D,Sprake C H S,Townsend R.Thermodynamic properties of organic oxygen compounds(ⅩⅪⅩ): Vapor pressure of diethyl ether [J].J. Chem. Thermodyn.,1972,4(2):247-254. |
| 91 | Ambrose D,Ellender J H,Sprake C H S,et al.Thermodynamic properties of organic oxygen compounds(ⅩLⅢ): Vapor pressures of some ethers [J].J. Chem. Thermodyn.,1976,8(2):165-178. |
| 92 | Ambrose D,Sprake C H S,Townsend R.Thermodynamic properties of organic oxygen compounds(ⅩⅩ ): Vapor pressure of acetone [J].J. Chem. Thermodyn.,1974,6(7):693-700. |
| 93 | Silberberg I H,Mcketta J J,Kobe K A.Compressibility of isopentane with the burnett apparatus [J].J. Chem. Eng. Data,1959,4(4):323-329. |
| 94 | Kobe K A,Crawford H R,Stephenson R W.Critical properties and vapor pressures of some ketones [J].Ind. Eng. Chem. Res.,1955,47:1767-1772. |
| 95 | Young S,Thomas G L.The vapour pressures, molecular volumes, and critical constants of ten of the lower esters [J]. J. Chem. Soc. Faraday Trans.,1893,63:1191-1262. |
| 96 | Polak J,Mertl I.Saturated vapor pressure of methyl acetate, ethyl acetate, propyl acetate, methyl propionate, and ethyl propionate [J].Collect. Czech. Chem. Commun.,1965,30(10):3526-3528. |
| 97 | Susial P,Susial R,Estupinan E J,et al.Determination and thermodynamic evaluation of isobaric VLE of methyl acetate or ethyl acetate with 2-propanol at 0.3 and 0.6 MPa [J].Fluid Phase Equilibr.,2014,375:1-10. |
| 98 | Mathews J H,Faville K E.Physical properties of a number of pure esters [J].J. Phys. Chem.,1918,22:1-22. |
| 99 | Lepori L,Matteoli E,Gianni P.Vapor pressure and its temperature dependence of 28 organic compounds: cyclic amines, cyclic ethers, and cyclic and open chain secondary alcohols [J].J. Chem. Eng. Data,2017,62(1):194-203. |
| 100 | Vinson C G,Martin J J.Heat of vaporization and vapor pressure of 1,4-dioxane [J].J. Chem. Eng. Data,1963,8(1):74-75. |
| 101 | Wagner W.The IAPWS formulation 1995 for the thermodynamic properties of ordinary water substance for general and scientific use [J]. J. Phys. Chem. Ref. Data,2002,31(2):387-535. |
| [1] | 康飞, 吕伟光, 巨锋, 孙峙. 废锂离子电池放电路径与评价研究[J]. 化工学报, 2023, 74(9): 3903-3911. |
| [2] | 尹刚, 李伊惠, 何飞, 曹文琦, 王民, 颜非亚, 向禹, 卢剑, 罗斌, 卢润廷. 基于KPCA和SVM的铝电解槽漏槽事故预警方法[J]. 化工学报, 2023, 74(8): 3419-3428. |
| [3] | 闫琳琦, 王振雷. 基于STA-BiLSTM-LightGBM组合模型的多步预测软测量建模[J]. 化工学报, 2023, 74(8): 3407-3418. |
| [4] | 郭雨莹, 敬加强, 黄婉妮, 张平, 孙杰, 朱宇, 冯君炫, 陆洪江. 稠油管道水润滑减阻及压降预测模型修正[J]. 化工学报, 2023, 74(7): 2898-2907. |
| [5] | 李艳辉, 丁邵明, 白周央, 张一楠, 于智红, 邢利梅, 高鹏飞, 王永贞. 非常规服役超临界锅炉的微纳尺度腐蚀动力学模型建立及应用[J]. 化工学报, 2023, 74(6): 2436-2446. |
| [6] | 于源, 陈薇薇, 付俊杰, 刘家祥, 焦志伟. 几何相似涡流空气分级机环形区流场变化规律研究及预测[J]. 化工学报, 2023, 74(6): 2363-2373. |
| [7] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [8] | 陈科, 杜理, 曾英, 任思颖, 于旭东. 四元体系LiCl+MgCl2+CaCl2+H2O 323.2 K相平衡研究及计算[J]. 化工学报, 2023, 74(5): 1896-1903. |
| [9] | 党玉荣, 莫春兰, 史科锐, 方颖聪, 张子杨, 李作顺. 综合评价模型联合遗传算法的混合工质ORC系统性能研究[J]. 化工学报, 2023, 74(5): 1884-1895. |
| [10] | 吴心远, 刘奇磊, 曹博渊, 张磊, 都健. Group2vec:基于无监督机器学习的基团向量表示及其物性预测应用[J]. 化工学报, 2023, 74(3): 1187-1194. |
| [11] | 张中秋, 李宏光, 石逸林. 基于人工预测调控策略的复杂化工过程多任务学习方法[J]. 化工学报, 2023, 74(3): 1195-1204. |
| [12] | 毛元敬, 杨智, 莫松平, 郭浩, 陈颖, 罗向龙, 陈健勇, 梁颖宗. C6~C10烷醇的SAFT-VR Mie状态方程参数回归及其热物性研究[J]. 化工学报, 2023, 74(3): 1033-1041. |
| [13] | 陈瑞哲, 程磊磊, 顾菁, 袁浩然, 陈勇. 纤维增强树脂复合材料化学回收技术研究进展[J]. 化工学报, 2023, 74(3): 981-994. |
| [14] | 史克年, 郑景元, 钱宇, 杨思宇. 基于马尔可夫链的蒸汽动力系统两阶段随机规划[J]. 化工学报, 2023, 74(2): 807-817. |
| [15] | 程文婷, 李杰, 徐丽, 程芳琴, 刘国际. AlCl3·6H2O在FeCl3、CaCl2、KCl及KCl–FeCl3溶液中溶解度的实验及预测[J]. 化工学报, 2023, 74(2): 642-652. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号