化工学报 ›› 2020, Vol. 71 ›› Issue (S1): 7-14.DOI: 10.11949/0438-1157.20190440
收稿日期:2019-04-26
修回日期:2019-07-28
出版日期:2020-04-25
发布日期:2020-04-25
通讯作者:
王云芳
作者简介:李攀(1992—),男,硕士研究生,基金资助:
Pan LI( ),Hui KONG,Zhuodong SONG,Zuoyi ZHANG,Yunfang WANG(
),Hui KONG,Zhuodong SONG,Zuoyi ZHANG,Yunfang WANG( )
)
Received:2019-04-26
Revised:2019-07-28
Online:2020-04-25
Published:2020-04-25
Contact:
Yunfang WANG
摘要:
聚甲氧基二甲醚(PODEn)是一种含氧量较高的化合物,近年来更是发展为一种优质的柴油添加剂。在101.3 kPa恒定压力下,使用改进的汽液平衡双循环Rose釜测定了甲醇-甲醛-PODE2三元体系汽液平衡数据。运用最大似然原理,在Aspen Plus软件中,分别采用NRTL、Wilson以及UNIQUAC三种活度系数模型对测定的三元汽液平衡数据进行回归,分别得到三个模型所对应的二元交互参数以及模拟计算值。通过对比模拟值与实验值,得到温度和气相组成的平均绝对偏差,分别小于1.10 K、0.0250和0.0240。三个模型的关联结果均适用于此体系,得到的二元交互参数能够应用于甲醇-甲醛-PODE2三元体系的精馏设计,为相关物系的工业分离优化奠定了数据基础。
中图分类号:
李攀, 孔慧, 宋卓栋, 张作毅, 王云芳. 甲醇-甲醛-聚甲氧基二甲醚三元体系汽液平衡[J]. 化工学报, 2020, 71(S1): 7-14.
Pan LI, Hui KONG, Zhuodong SONG, Zuoyi ZHANG, Yunfang WANG. Vapor-liquid equilibrium for methanol-formaldehyde-polyoxymethylene dimethyl ethers ternary system[J]. CIESC Journal, 2020, 71(S1): 7-14.
| 仪器名称 | 规格型号 | 生产厂家 |
|---|---|---|
| 改进的Rose釜 | 玻璃制双循环 | 北京玻璃仪器加工厂 |
| 精密温度计 | 1/20 | 北京玻璃研究院 |
| 电子分析天平 | AL204 | METTLER TOLEDO(上海)有限公司 |
| 气相色谱仪 | 安捷伦GC7890A | 美国安捷伦 |
| 均相反应器 | KLJX-12A | 烟台科立化工设备有限公司 |
| 电压调节器 | TDGC3-500W | 上海征西电气科技有限公司 |
| 烘箱 | DHG-9053A | 上海浦东荣丰科学仪器有限公司 |
表1 实验仪器
Table 1 Experimental instruments
| 仪器名称 | 规格型号 | 生产厂家 |
|---|---|---|
| 改进的Rose釜 | 玻璃制双循环 | 北京玻璃仪器加工厂 |
| 精密温度计 | 1/20 | 北京玻璃研究院 |
| 电子分析天平 | AL204 | METTLER TOLEDO(上海)有限公司 |
| 气相色谱仪 | 安捷伦GC7890A | 美国安捷伦 |
| 均相反应器 | KLJX-12A | 烟台科立化工设备有限公司 |
| 电压调节器 | TDGC3-500W | 上海征西电气科技有限公司 |
| 烘箱 | DHG-9053A | 上海浦东荣丰科学仪器有限公司 |
| 试剂名称 | 规格 | 标牌纯度/%(质量) | 生产厂家 | GC纯度/%(质量) |
|---|---|---|---|---|
| 甲醇 | 分析纯 | ≥99.5 | 国药集团 | 99.81 |
| PODE1 | 分析纯 | ≥99.7 | 国药集团 | 99.83 |
| PODE2 | — | — | 自制 | 99.49 |
| 多聚甲醛 | — | — | 自制 | 99.43 |
表2 实验试剂
Table 2 Experimental reagents
| 试剂名称 | 规格 | 标牌纯度/%(质量) | 生产厂家 | GC纯度/%(质量) |
|---|---|---|---|---|
| 甲醇 | 分析纯 | ≥99.5 | 国药集团 | 99.81 |
| PODE1 | 分析纯 | ≥99.7 | 国药集团 | 99.83 |
| PODE2 | — | — | 自制 | 99.49 |
| 多聚甲醛 | — | — | 自制 | 99.43 |
| No. | Texp/K | x1,exp | x2,exp | y1,exp | y2,exp | Test/K | y1,est | y2,est | ΔT/K | Δy1 | Δy2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 368.79 | 0.0305 | 0.9441 | 0.0619 | 0.9031 | 368.77 | 0.0532 | 0.9171 | -0.03 | -0.0088 | 0.0140 |
| 2 | 366.99 | 0.0425 | 0.9327 | 0.0996 | 0.8694 | 367.37 | 0.0778 | 0.8966 | 0.38 | -0.0219 | 0.0272 |
| 3 | 365.83 | 0.0548 | 0.9200 | 0.1308 | 0.8400 | 366.02 | 0.1024 | 0.8739 | 0.20 | -0.0284 | 0.0339 |
| 4 | 364.33 | 0.0689 | 0.9035 | 0.1623 | 0.8102 | 364.92 | 0.1262 | 0.8502 | 0.59 | -0.0361 | 0.0399 |
| 5 | 362.00 | 0.0846 | 0.8900 | 0.2015 | 0.7748 | 362.56 | 0.1673 | 0.8127 | 0.56 | -0.0342 | 0.0379 |
| 6 | 360.43 | 0.0985 | 0.8746 | 0.2358 | 0.7413 | 361.10 | 0.1936 | 0.7857 | 0.67 | -0.0421 | 0.0444 |
| 7 | 359.54 | 0.1070 | 0.8654 | 0.2543 | 0.7227 | 360.21 | 0.2100 | 0.7690 | 0.67 | -0.0443 | 0.0464 |
| 8 | 357.36 | 0.1350 | 0.8372 | 0.2937 | 0.6846 | 357.28 | 0.2683 | 0.7116 | -0.08 | -0.0255 | 0.0269 |
| 9 | 355.44 | 0.1548 | 0.8167 | 0.3330 | 0.6479 | 355.22 | 0.3103 | 0.6704 | -0.22 | -0.0226 | 0.0224 |
| 10 | 352.66 | 0.1959 | 0.7752 | 0.3934 | 0.5875 | 351.99 | 0.3797 | 0.6004 | -0.67 | -0.0137 | 0.0129 |
| 11 | 351.32 | 0.2143 | 0.7589 | 0.4264 | 0.5567 | 350.30 | 0.4164 | 0.5657 | -1.01 | -0.0100 | 0.0090 |
| 12 | 350.62 | 0.2237 | 0.7474 | 0.4416 | 0.5429 | 349.71 | 0.4319 | 0.5509 | -0.91 | -0.0097 | 0.0080 |
| 13 | 350.16 | 0.2338 | 0.7373 | 0.4529 | 0.5312 | 349.18 | 0.4444 | 0.5381 | -0.98 | -0.0086 | 0.0069 |
| 14 | 348.16 | 0.2699 | 0.7023 | 0.4931 | 0.4925 | 347.07 | 0.4945 | 0.4894 | -1.10 | 0.0014 | -0.0030 |
| 15 | 347.44 | 0.2881 | 0.6852 | 0.5087 | 0.4763 | 346.30 | 0.5132 | 0.4705 | -1.14 | 0.0045 | -0.0058 |
| 16 | 346.45 | 0.3197 | 0.6542 | 0.5328 | 0.4533 | 345.11 | 0.5467 | 0.4381 | -1.34 | 0.0139 | -0.0152 |
| 17 | 369.39 | 0.0279 | 0.9420 | 0.0476 | 0.9003 | 368.91 | 0.0439 | 0.9162 | -0.48 | -0.0037 | 0.0160 |
| 18 | 368.79 | 0.0388 | 0.9280 | 0.0657 | 0.8867 | 368.37 | 0.0587 | 0.9015 | -0.42 | -0.0070 | 0.0148 |
| 19 | 367.37 | 0.0511 | 0.9110 | 0.0959 | 0.8565 | 367.63 | 0.0733 | 0.8839 | 0.27 | -0.0226 | 0.0273 |
| 20 | 364.11 | 0.0824 | 0.8754 | 0.1618 | 0.7934 | 365.28 | 0.1194 | 0.8405 | 1.17 | -0.0424 | 0.0471 |
| 21 | 362.15 | 0.1054 | 0.8515 | 0.2048 | 0.7549 | 363.33 | 0.1585 | 0.8046 | 1.17 | -0.0463 | 0.0498 |
| 22 | 359.54 | 0.1348 | 0.8219 | 0.2652 | 0.7016 | 360.11 | 0.2214 | 0.7476 | 0.57 | -0.0438 | 0.0460 |
| 23 | 357.24 | 0.1613 | 0.7949 | 0.3156 | 0.6531 | 357.62 | 0.2700 | 0.6986 | 0.38 | -0.0456 | 0.0455 |
| 24 | 356.03 | 0.1815 | 0.7759 | 0.3449 | 0.6257 | 355.95 | 0.3084 | 0.6621 | -0.08 | -0.0365 | 0.0365 |
| 25 | 354.74 | 0.2000 | 0.7558 | 0.3694 | 0.5984 | 354.66 | 0.3346 | 0.6335 | -0.08 | -0.0349 | 0.0351 |
| 26 | 353.86 | 0.2137 | 0.7419 | 0.3885 | 0.5837 | 353.64 | 0.3608 | 0.6094 | -0.23 | -0.0278 | 0.0257 |
表3 NRTL模型对甲醇-甲醛-PODE2三元体系的模拟结果
Table 3 Simulation results for methanol-formaldehyde-PODE2 ternary system using NRTL model
| No. | Texp/K | x1,exp | x2,exp | y1,exp | y2,exp | Test/K | y1,est | y2,est | ΔT/K | Δy1 | Δy2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 368.79 | 0.0305 | 0.9441 | 0.0619 | 0.9031 | 368.77 | 0.0532 | 0.9171 | -0.03 | -0.0088 | 0.0140 |
| 2 | 366.99 | 0.0425 | 0.9327 | 0.0996 | 0.8694 | 367.37 | 0.0778 | 0.8966 | 0.38 | -0.0219 | 0.0272 |
| 3 | 365.83 | 0.0548 | 0.9200 | 0.1308 | 0.8400 | 366.02 | 0.1024 | 0.8739 | 0.20 | -0.0284 | 0.0339 |
| 4 | 364.33 | 0.0689 | 0.9035 | 0.1623 | 0.8102 | 364.92 | 0.1262 | 0.8502 | 0.59 | -0.0361 | 0.0399 |
| 5 | 362.00 | 0.0846 | 0.8900 | 0.2015 | 0.7748 | 362.56 | 0.1673 | 0.8127 | 0.56 | -0.0342 | 0.0379 |
| 6 | 360.43 | 0.0985 | 0.8746 | 0.2358 | 0.7413 | 361.10 | 0.1936 | 0.7857 | 0.67 | -0.0421 | 0.0444 |
| 7 | 359.54 | 0.1070 | 0.8654 | 0.2543 | 0.7227 | 360.21 | 0.2100 | 0.7690 | 0.67 | -0.0443 | 0.0464 |
| 8 | 357.36 | 0.1350 | 0.8372 | 0.2937 | 0.6846 | 357.28 | 0.2683 | 0.7116 | -0.08 | -0.0255 | 0.0269 |
| 9 | 355.44 | 0.1548 | 0.8167 | 0.3330 | 0.6479 | 355.22 | 0.3103 | 0.6704 | -0.22 | -0.0226 | 0.0224 |
| 10 | 352.66 | 0.1959 | 0.7752 | 0.3934 | 0.5875 | 351.99 | 0.3797 | 0.6004 | -0.67 | -0.0137 | 0.0129 |
| 11 | 351.32 | 0.2143 | 0.7589 | 0.4264 | 0.5567 | 350.30 | 0.4164 | 0.5657 | -1.01 | -0.0100 | 0.0090 |
| 12 | 350.62 | 0.2237 | 0.7474 | 0.4416 | 0.5429 | 349.71 | 0.4319 | 0.5509 | -0.91 | -0.0097 | 0.0080 |
| 13 | 350.16 | 0.2338 | 0.7373 | 0.4529 | 0.5312 | 349.18 | 0.4444 | 0.5381 | -0.98 | -0.0086 | 0.0069 |
| 14 | 348.16 | 0.2699 | 0.7023 | 0.4931 | 0.4925 | 347.07 | 0.4945 | 0.4894 | -1.10 | 0.0014 | -0.0030 |
| 15 | 347.44 | 0.2881 | 0.6852 | 0.5087 | 0.4763 | 346.30 | 0.5132 | 0.4705 | -1.14 | 0.0045 | -0.0058 |
| 16 | 346.45 | 0.3197 | 0.6542 | 0.5328 | 0.4533 | 345.11 | 0.5467 | 0.4381 | -1.34 | 0.0139 | -0.0152 |
| 17 | 369.39 | 0.0279 | 0.9420 | 0.0476 | 0.9003 | 368.91 | 0.0439 | 0.9162 | -0.48 | -0.0037 | 0.0160 |
| 18 | 368.79 | 0.0388 | 0.9280 | 0.0657 | 0.8867 | 368.37 | 0.0587 | 0.9015 | -0.42 | -0.0070 | 0.0148 |
| 19 | 367.37 | 0.0511 | 0.9110 | 0.0959 | 0.8565 | 367.63 | 0.0733 | 0.8839 | 0.27 | -0.0226 | 0.0273 |
| 20 | 364.11 | 0.0824 | 0.8754 | 0.1618 | 0.7934 | 365.28 | 0.1194 | 0.8405 | 1.17 | -0.0424 | 0.0471 |
| 21 | 362.15 | 0.1054 | 0.8515 | 0.2048 | 0.7549 | 363.33 | 0.1585 | 0.8046 | 1.17 | -0.0463 | 0.0498 |
| 22 | 359.54 | 0.1348 | 0.8219 | 0.2652 | 0.7016 | 360.11 | 0.2214 | 0.7476 | 0.57 | -0.0438 | 0.0460 |
| 23 | 357.24 | 0.1613 | 0.7949 | 0.3156 | 0.6531 | 357.62 | 0.2700 | 0.6986 | 0.38 | -0.0456 | 0.0455 |
| 24 | 356.03 | 0.1815 | 0.7759 | 0.3449 | 0.6257 | 355.95 | 0.3084 | 0.6621 | -0.08 | -0.0365 | 0.0365 |
| 25 | 354.74 | 0.2000 | 0.7558 | 0.3694 | 0.5984 | 354.66 | 0.3346 | 0.6335 | -0.08 | -0.0349 | 0.0351 |
| 26 | 353.86 | 0.2137 | 0.7419 | 0.3885 | 0.5837 | 353.64 | 0.3608 | 0.6094 | -0.23 | -0.0278 | 0.0257 |
| 模型方程 | 平衡温度/K | 甲醇气相浓度 | PODE2气相浓度 | |||
|---|---|---|---|---|---|---|
平均 绝对偏差 | 最大 绝对偏差 | 平均 绝对偏差 | 最大 绝对偏差 | 平均 绝对偏差 | 最大 绝对偏差 | |
| Wilson | 0.96 | 2.32 | 0.0240 | 0.0584 | 0.0235 | 0.0583 |
| NRTL | 1.08 | 2.74 | 0.0246 | 0.0552 | 0.0237 | 0.0554 |
| UNIQUAC | 0.95 | 2.30 | 0.0243 | 0.0646 | 0.0238 | 0.0597 |
表4 三个模型对于三元体系的模拟结果对比
Table 4 Comparison of simulation results for ternary system using three models
| 模型方程 | 平衡温度/K | 甲醇气相浓度 | PODE2气相浓度 | |||
|---|---|---|---|---|---|---|
平均 绝对偏差 | 最大 绝对偏差 | 平均 绝对偏差 | 最大 绝对偏差 | 平均 绝对偏差 | 最大 绝对偏差 | |
| Wilson | 0.96 | 2.32 | 0.0240 | 0.0584 | 0.0235 | 0.0583 |
| NRTL | 1.08 | 2.74 | 0.0246 | 0.0552 | 0.0237 | 0.0554 |
| UNIQUAC | 0.95 | 2.30 | 0.0243 | 0.0646 | 0.0238 | 0.0597 |
| 方程 | 体系 | aij | aji | bij | bji | cij |
|---|---|---|---|---|---|---|
| Wilson | 甲醇-甲醛 | 20.7721585 | 6.5176 | -1275.03466 | -875.896626 | 0 |
| 甲醇-PODE2 | 9.41749887 | 29.1792322 | -6476.11242 | -10000 | 0 | |
| 甲醛-PODE2 | -0.043122698 | 30.4888446 | -370.184734 | -10000 | 0 | |
| NRTL | 甲醇-甲醛 | -27.5292688 | -22.8532058 | 10000 | 6862.58382 | 0.3 |
| 甲醇-PODE2 | -9.68694324 | 3.15389659 | 3321.78975 | -813.924515 | 0.3 | |
| 甲醛-PODE2 | -29.817718 | -3.97475484 | 10000 | 1420.3292 | 0.3 | |
| UNIQUAC | 甲醇-甲醛 | 27.8659376 | 17.0564607 | -10000 | -5171.74989 | 0 |
| 甲醇-PODE2 | -2.88017095 | 7.4251551 | 1021.65838 | -2793.07369 | 0 | |
| 甲醛-PODE2 | 28.3279998 | -0.18584951 | -10000 | 291.98213 | 0 |
表5 甲醇-甲醛-PODE2三元体系的二元交互参数
Table 5 Binary interaction parameters for methanol - formaldehyde -PODE2 ternary system
| 方程 | 体系 | aij | aji | bij | bji | cij |
|---|---|---|---|---|---|---|
| Wilson | 甲醇-甲醛 | 20.7721585 | 6.5176 | -1275.03466 | -875.896626 | 0 |
| 甲醇-PODE2 | 9.41749887 | 29.1792322 | -6476.11242 | -10000 | 0 | |
| 甲醛-PODE2 | -0.043122698 | 30.4888446 | -370.184734 | -10000 | 0 | |
| NRTL | 甲醇-甲醛 | -27.5292688 | -22.8532058 | 10000 | 6862.58382 | 0.3 |
| 甲醇-PODE2 | -9.68694324 | 3.15389659 | 3321.78975 | -813.924515 | 0.3 | |
| 甲醛-PODE2 | -29.817718 | -3.97475484 | 10000 | 1420.3292 | 0.3 | |
| UNIQUAC | 甲醇-甲醛 | 27.8659376 | 17.0564607 | -10000 | -5171.74989 | 0 |
| 甲醇-PODE2 | -2.88017095 | 7.4251551 | 1021.65838 | -2793.07369 | 0 | |
| 甲醛-PODE2 | 28.3279998 | -0.18584951 | -10000 | 291.98213 | 0 |

图1 Wilson模型对于甲醇-甲醛-PODE2体系的模拟值与实验值的对比
Fig.1 Comparison between estimated value and experimental value for methanol - formaldehyde-PODE2 system using Wilson model
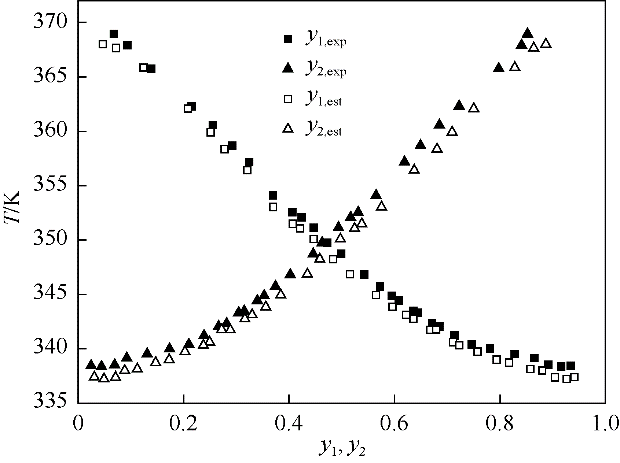
图2 NRTL模型对于甲醇-甲醛-PODE2体系的模拟值与实验值的对比
Fig.2 Comparison between estimated value and experimental value for methanol - formaldehyde-PODE2 system using NRTL model

图3 UNIQUAC模型对于甲醇-甲醛-PODE2体系的模拟值与实验值的对比
Fig.3 Comparison between estimated value and experimental value for methanol - formaldehyde-PODE2 system using UNIQUAC model
| 1 | 李振光, 袁建团. 中国柴油需求趋势分析[J]. 国际石油经济, 2015, 23(9): 88-93. |
| Li Z G, Yuan J G. China s diesel demand[J]. International Petroleum Economics, 2015, 23(9): 88-93. | |
| 2 | 石华. 我国石油对外依存度首破60%[J]. 石油库与加油站, 2016, 25(1): 25. |
| Shi H. China s oil external dependence first broke 60%[J]. Oil Depot and Gas Station, 2016, 25(1): 25. | |
| 3 | 焦瑞. 如何认识我国原油对外依存度逐年攀升[J]. 炼油技术与工程, 2017, (4): 45. |
| Jiao R. How to understand China s crude oil dependence increasing year by year[J]. Refining Technology and Engineering, 2017, (4): 45. | |
| 4 | 钱伯章. 到2020年柴油需求将超过汽油[J]. 润滑油与燃料, 2014, (z1): 34. |
| Qian B Z. Demand for diesel will exceed demand for gasoline by 2020[J]. Lube and Fuel, 2014, (z1): 34. | |
| 5 | 李俊涛. 添加剂对柴油质量影响的研究[D]. 武汉: 武汉工程大学, 2016. |
| Li J T. Study on the effect of additives on diesel oil quality[D]. Wuhan: Wuhan Institute of Technology, 2016. | |
| 6 | 王志, 刘浩业, 张俊, 等. 聚甲氧基二甲醚与柴油混合燃料的燃烧与排放特性[J]. 汽车安全与节能学报, 2015, 6(2): 191-197. |
| Wang Z, Liu H Y, Zhang J, et al. Combustion and emission characteristics of diesel engines fuelled by polyoxymethylene dimethyl ethers (PODEn)[J]. Journal of Automotive Safety and Energy, 2015, 6(2): 191-197. | |
| 7 | Pellegrini L, Marchionna M, Patrini R, et al. Combustion behaviour and emission performance of neat and blended polyoxymethylene dimethyl ethers in a light-duty diesel engine[R]. SAETechnical Paper, 2012. |
| 8 | Pellegrini L, Marchionna M, Patrini R, et al. Emission performance of neat and blended polyoxymethylene dimethyl ethers in an old light-duty diesel car[R]. SAETechnical Paper, 2013. |
| 9 | 张信伟, 李杰, 倪向前, 等. 聚甲氧基二甲醚合成技术的产业化进展[J]. 化工进展, 2016, 35(7): 2293-2298. |
| Zhang X W, Li J, Ni X Q, et al. Industrialization progress of polyoxymethylene dimethyl ethers synthesis technology[J]. Chemical Industry and Engineering Progress, 2016, 35(7): 2293-2298. | |
| 10 | 杨丰科, 王俊伟. 柴油添加剂聚甲氧基二甲醚的合成研究进展[J]. 应用化工, 2012, 41(10): 1803-1806. |
| Yang F K, Wang J W. Progress on the synthesis of polyoxymethylene dimethyl ethers as component of tailored diesel fuel[J]. Applied Chemical Industry, 2012, 41(10): 1803-1806. | |
| 11 | 史高峰, 陈英赞, 陈学福, 等. 聚甲氧基二甲醚研究进展[J]. 天然气化工, 2012, 37(2): 74-78. |
| Shi G F, Chen Y Z, Chen X F, et al. Research progress in polyoxymethylene dimethyl ethers[J]. Natural Gas Chemical Industry, 2012, 37(2): 74-78. | |
| 12 | 张建强, 唐斌, 刘殿华, 等. 聚甲氧基二甲醚合成研究现状[J]. 煤化工, 2013, (1): 41-43. |
| Zhang J Q, Tang B, Liu D H, et al. Research progress of polyoxymethylene dimethyl ethers synthesis[J]. Coal Chemical Industry, 2013, (1): 41-43. | |
| 13 | Burger J, Siegert M, Ströfer E, et al. Poly (oxymethylene) dimethyl ethers as components of tailored diesel fuel: properties, synthesis and purification concepts[J]. Fuel, 2010, 89(11): 3315-3319. |
| 14 | Burger J, Ströfer E, Hasse H. Chemical equilibrium and reaction kinetics of the heterogeneously catalyzed formation of poly(oxymethylene) dimethyl ethers from methylal and trioxane[J]. Industrial & Engineering Chemistry Research, 2012, 51(39): 12751-12761. |
| 15 | Burger J, Hasse H. Multi-objective optimization using reduced models in conceptual design of a fuel additive production process[J]. Chemical Engineering Science, 2013, 99: 118-126. |
| 16 | Burger J, Ströfer E, Hasse H. Production process for diesel fuel components polyoxymethylene dimethyl ethers from methane-based products by hierarchical optimization with varying model depth[J]. Chemical Engineering Research and Design, 2013, 91(12): 2648-2662. |
| 17 | 孙兰义. 化工过程流程模拟实训: Aspen Plus教程[M]. 北京: 化学工业出版社, 2012. |
| Sun L Y. Chemical Process Process Simulation Training: Aspen Plus Tutorial[M]. Beijing: Chemical Industry Press, 2012. | |
| 18 | 张治山, 杨超龙. Aspen Plus在化工中的应用[J]. 广东化工, 2012, 39(3): 77-78. |
| Zhang Z S, Yang C L. Application of Aspen Plus in chemical engineering[J]. Guangdong Chemical Industry, 2012, 39(3): 77-78. | |
| 19 | More R K, Bulasara V K, Uppaluri R, et al. Optimization of crude distillation system using Aspen Plus: effect of binary feed selection on grass-root design[J]. Chemical Engineering Research & Design, 2010, 88(2): 121-134. |
| 20 | Ramzan N, Ashraf A, Naveed S, et al. Simulation of hybrid biomass gasification using Aspen plus: a comparative performance analysis for food, municipal solid and poultry waste[J]. Biomass & Bioenergy, 2011, 35(9): 3962-3969. |
| 21 | Doherty W, Reynolds A, Kennedy D. Computer simulation of a biomass gasification-solid oxide fuel cell power system using Aspen Plus[J]. Energy, 2010, 35(12): 4545-4555. |
| 22 | 周怀申, 韩世钧. 一种新的循环法汽液平衡釜——改进的Rose釜[J]. 化学工程, 1980, (5): 23-26. |
| Zhou H S, Han S J. A new kind of vapor liquid balance kettle of circulating method — improved Rose kettle[J]. Chemical Engineering(China), 1980, (5): 23-26. | |
| 23 | 朱云庆.醋酸物系相平衡研究[D]. 青岛: 中国石油大学, 2008. |
| Zhu Y Q. Study on phase equilibrium of acetic acid[D]. Qingdao: China University of Petroleum, 2008. | |
| 24 | 任海鸥, 周金波, 李长明, 等. 常压下叔丁醇-水-乙二醇体系汽液平衡测定与关联[J]. 化学工程, 2017, 45(9): 35-38. |
| Ren H O, Zhou J B, Li C M, et al. Determination and correlation of vapor - liquid equilibrium of tert-butanol - water - glycol system under atmospheric pressure[J]. Chemical Engineering(China), 2017, 45(9): 35-38. | |
| 25 | 周航. 乙醇-丙酸乙酯-对二甲苯等压汽液相平衡研究[D]. 天津: 天津大学, 2017. |
| Zhou H. Study on vapor-liquid equilibrium of ethanol-ethyl propionate -p-xylene at constant pressure[D]. Tianjin: Tianjin University, 2017. | |
| 26 | 曹玲, 徐威震, 李学琴, 等. 甲醇+碳酸二甲酯+二乙二醇三元物系等压汽液平衡数据的测定与关联[J]. 石油化工, 2017, 46(9): 74-79. |
| Cao L, Xu W Z, Li X Q, et al. Isobaric vapor-liquid equilibrium for methanol+dimethyl carbonate+ diethylene glycol system[J]. Petrochemical Technology, 2017, 46(9): 74-79. | |
| 27 | Nagahama K. VLE measurements at elevated pressures for process development [J]. Fluid Phase Equilibria, 1996, 116(1): 361-372. |
| 28 | Fornari R E, Alessi P, Kikic I. High-pressure fluid-phase equilibria: experimental methods and systems investigated(1978—1987)[J]. Fluid Phase Equilibria, 1990, 57: 1-33. |
| 29 | Dohrn R, Brunner G. High-pressure fluid-phase equilibria: experimental methods and systems investigated (1988—1993) [J]. Fluid Phase Equilibria, 1995, 106(1): 213-282. |
| 30 | 王琦. 双循环加压汽液相平衡装置的建立与校核[J]. 石油学报, 1988, 4(2): 50-56. |
| Wang Q. Establishment and verification of double cycle pressurized vapor liquid phase balance device[J]. Acta Petroleum Sinica, 1988, 4(2): 50-56. |
| [1] | 汪尔奇, 彭书舟, 杨震, 段远源. 含HFO混合体系气液相平衡的理论模型评价[J]. 化工学报, 2023, 74(8): 3216-3225. |
| [2] | 杨菲菲, 赵世熙, 周维, 倪中海. Sn掺杂的In2O3催化CO2选择性加氢制甲醇[J]. 化工学报, 2023, 74(8): 3366-3374. |
| [3] | 李凯旋, 谭伟, 张曼玉, 徐志豪, 王旭裕, 纪红兵. 富含零价钴活性位点的钴氮碳/活性炭设计及甲醛催化氧化应用研究[J]. 化工学报, 2023, 74(8): 3342-3352. |
| [4] | 李贵贤, 曹阿波, 孟文亮, 王东亮, 杨勇, 周怀荣. 耦合固体氧化物电解槽的CO2制甲醇过程设计与评价研究[J]. 化工学报, 2023, 74(7): 2999-3009. |
| [5] | 姚晓宇, 沈俊, 李健, 李振兴, 康慧芳, 唐博, 董学强, 公茂琼. 流体气液临界参数测量方法研究进展[J]. 化工学报, 2023, 74(5): 1847-1861. |
| [6] | 吕阳光, 左培培, 杨正金, 徐铜文. 三嗪框架聚合物膜用于有机纳滤甲醇/正己烷分离[J]. 化工学报, 2023, 74(4): 1598-1606. |
| [7] | 沈辰阳, 孙楷航, 张月萍, 刘昌俊. 二氧化碳加氢合成甲醇氧化铟及其负载金属催化剂研究进展[J]. 化工学报, 2023, 74(1): 145-156. |
| [8] | 戴文华, 辛忠. Si掺杂对Cu/ZrO2催化CO2加氢制甲醇性能的影响[J]. 化工学报, 2022, 73(8): 3586-3596. |
| [9] | 张军, 胡升, 顾菁, 袁浩然, 陈勇. 甲醇体系电镀污泥衍生磁性多金属材料催化糠醛加氢转化[J]. 化工学报, 2022, 73(7): 2996-3006. |
| [10] | 李丽媛, 王建强, 陈奕, 郭友娣, 周健, 刘志成, 王仰东, 谢在库. 甲醇制丙烯反应中ZSM-5分子筛催化剂积炭失活介尺度机制研究[J]. 化工学报, 2022, 73(6): 2669-2676. |
| [11] | 张家仁, 刘海超. 大豆油与甲醇酯交换反应体系的相平衡研究[J]. 化工学报, 2022, 73(5): 1920-1929. |
| [12] | 孟文亮, 李贵贤, 周怀荣, 李婧玮, 王健, 王可, 范学英, 王东亮. 绿氢重构的粉煤气化煤制甲醇近零碳排放工艺研究[J]. 化工学报, 2022, 73(4): 1714-1723. |
| [13] | 门文欣, 彭庆收, 桂霞. 不同季铵盐作用下的CO2水合物相平衡[J]. 化工学报, 2022, 73(4): 1472-1482. |
| [14] | 高欢, 丁国良, 周发贤, 庄大伟. R410A制冷剂在润滑油中的动态析出特性的研究[J]. 化工学报, 2022, 73(3): 1054-1062. |
| [15] | 王建, 雷子萱, 姚家钰, 李建, 刘育红. 对苯二甲醛酚醛树脂的制备及其固化动力学研究[J]. 化工学报, 2022, 73(3): 1403-1415. |
| 阅读次数 | ||||||
|
全文 |
|
|||||
|
摘要 |
|
|||||
 京公网安备 11010102001995号
京公网安备 11010102001995号